Introduction
Worldwide, more than 550,000 cases and 380,000 deaths occur annually from head and neck squamous cell carcinoma (HNSCC).1 In the United States, 63,000 patients are diagnosed annually, and about 13,000 die from the disease.2 The majority are related to tobacco and alcohol use, but the number of high-risk human papillomavirus (HPV)-associated oropharynx cancers is increasing; these are often identified in clinical trials via staining for the surrogate marker p16. Tumors that are p16-positive (+) have a more favorable prognosis and may require less intense treatment.3,4
Patients with HPV-associated cancers are significantly younger, have tumors typically in the tonsillar region or base of tongue, and tend to present with early-stage primary tumors with an increased risk of advanced nodal involvement when compared with HPV-negative (-) patients.5 HPV-associated tumors are pathologically distinct, with lymphocyte infiltration in the stroma and in tumor nests.6 Despite advances in treatment, there is still a relatively poor 10-month median overall survival (OS) for recurrent/metastatic (r/m) disease,7 with some evidence that survival is greater for HPV-associated than HPV- disease.8
HPV-associated HNSCC can have mutations from expression of apolipoprotein B mRNA editing enzymes or catalytic poly- peptide-like cytidine deaminases, which are responsible for DNA editing and cause mutation clusters in multiple tumor types.9 HPV- HNSCC tends to have mutations caused by smoking. The overall mutation burden is similar in both types of HNSCC, but the specific mutational composition is very different; 5 distinct subtypes have been identified.10-12
HNSCC is associated with multiple alterations in the immune system, potentially resulting in depressed antitumor immunity. These alterations include expression of immune checkpoint molecules, which increase the proportion of immunosuppressive regulatory T cells (Tregs) in the circulation and within the tumor microenvironment,13-15 cause dysregulation of T-cell function,16 alter cytokine production17 and myeloid dendritic cell (DC) function,18 and decrease the number of natural killer (NK) cells.19,20 Therapies that overcome these immune checkpoints and restore immune function have demonstrated activity in HNSCC, and combination strategies—of more than 1 immunotherapy, or of immunotherapy with conventional therapy—are under active investigation. Immunotherapies currently being studied include checkpoint inhibitors, therapeutic vaccines, costimulatory agonists, adoptive T-cell therapies, and mono- clonal antibodies (mAbs). This review will focus on the checkpoint inhibitors, combinations of checkpoint inhibitors, and therapeutic vaccines, as well as costimulatory agonists.
Immune Checkpoints
Tregs are a subset of CD4+/CD25+ T cells21 that express the canonical transcription factor FOXP3.22,23 Tregs compose 5% to 10% of CD4+ T cells in the peripheral blood of healthy persons,24 but in patients with a malignancy, peripheral blood mononuclear cells (PBMCs) may contain up to 25% to 30% of Tregs.25 Tregs increase has been documented across patients with a variety of malignancies,14,26,27 including HNSCC.28 Tregs suppress NK cells, DCs, and B-cell function,29,30 and are significantly enriched in tumor-infiltrating lymphocytes (TILs) com- pared with autologous PBMCs in patients with HNSCC,31 dampening antitumor immunity.32 Furthermore, they release suppressive cytokines and express cytotoxic T-lymphocyte–associated protein 4 (CTLA-4).33,34 Tregs number is increased in HNSCC patients before and after treat- ment.35 Interestingly, patients with advanced disease and those who have undergone treatment with curative intent have the same proportions of Tregs in the periphery, suggesting an immune dysregulation by the tumor that does not resolve after treatment.14
The regulatory PD-1 receptor is expressed on activated T cells. PD-1 signaling is activated upon binding by the ligands, PD-L1 and PD-L2. PD-L1 (also known as B7-H1) is a transmembrane glycoprotein that may be expressed on tumor cells or tumor-infiltrating immune cells, while PD-L2 is primarily expressed on macrophages and DCs. The binding of PD-1 to PD-L1 causes downregulation of the T-cell response.36 PD-L1 expression reduces T-cell activation by binding to PD-1 and CD80, reducing CD28 co-stimulation.37,38 CTLA-4, another negative regulator of T-cell activation, is present on the cell surface of CD4+ and CD8+ T cells, where it binds to CD80 and CD86 (B7-1 and B7-2) to reduce CD28-mediated T-cell activation.39
Knowing the normal distribution of PD-1 and PD-L1/2 is import- ant in understanding the adverse effects of the checkpoint inhibitors. PD-1 is expressed not only on activated T cells, but also on double- negative (CD4-/CD8-) T cells in the thymus, activated NK T cells, B cells, monocytes, and immature Langerhans cells.40 PD-L1 is expressed on a diverse number of cell types, including antigen-presenting cells (APCs), vascular endothelial cells, pancreatic islet cells, and placenta, testes, and eye.40,41 PD-L2 is expressed on DCs and macrophages. PD-L1 is expressed in HNSCC in 50% to 60% of cases42 and is more common in those patients with HPV-associated HNSCC.43 The check- point inhibitors thus have a biological basis for use in HNSCC.44-47
Checkpoint Inhibitors in the Recurrent/Metastatic Setting
Pembrolizumab is a humanized anti–PD-1 antibody. It was tested in patients with r/m HNSCC who were PD-L1+ in the KEYNOTE-012 trial.48 The initial cohort of the study included patients with any level of PD-L1 expression (>1% of tumor or immune cells) by immunohistochemistry (IHC). A 6-gene panel of interferon (IFN)-gamma–related genes, previously shown to be predictive of clinical outcome in the KEYNOTE-001 study,49 was also measured in tumor samples. Patients received pembrolizumab 10 mg/kg intravenously every 2 weeks. There were 104 patients screened, of whom 81 (78%) were PD-L1+; of these, 60 patients were treated. Forty-nine of these 60 patients discontinued treatment, 35 because of progressive disease. Of the 60 that were treated, both p16+ (38%) and p16- (62%) patients were enrolled. Seventeen percent of patients experienced grade 3 to 4 drug-related adverse events (AEs), of which the most common were transaminitis and hyponatremia. The most common low-grade AEs were fatigue, pruritus, nausea, decreased appetite, and rash. Overall response rate (ORR) by central review was 18%, with a numerically higher response rate of 25% in p16+ than in p16- patients (19%). The median OS was 13 months (95% CI, 5 to not reached) in the intention-to-treat population (n = 61) and 8 months (95% CI, 4 to not reached) for the p16- subgroup; it was not reached for the p16+ patients (95% CI, 8 to not reached); and the median duration of response among responders was 53 weeks. Higher levels of PD-L1 and IFN-gamma–related gene expression signature correlated with response.
Criticisms exist of this pilot study. The first is that there were several patients with primary tumor sites that are typically not included in clinical trials, including 9 (15%) whose site was “other or unknown primary” and 4 (7%) whose site was the nasal cavity.50 Nasopharyngeal cancer has been previously excluded in phase III trials of chemotherapy in the r/m setting.7,51 It has unique histology characterized by substantial lymphocytic infiltration in the primary tumor,52,53 and new data suggest that high PD-L1 levels in these tumors correlate with reduced survival.54 The pilot study also included 7 (12%) patients who had not previously received therapy for r/m disease. In the CheckMate 141 trial discussed below, the OS benefit with nivolumab was most prominent in those patients who had not received cetuximab previously, with hazard ratio (HR) = 0.55 (95% CI, 0.35-0.81) compared with HR = 0.81 (95% CI, 0.57-1.15) for those who had, suggesting that those who have not had treatment before are more likely to respond. The pattern of care of first- or second-line treatment was not uniform; of the patients who were previously treated, 63% had received cetuximab and platinum therapy, while 25% had received platinum without cetuximab. Finally, HPV status was determined by the use of p16 IHC as a surrogate marker, and it has been shown that there are significant limitations of p16 as a surrogate marker in nonoropharyngeal sites.
However, in this trial, only 2 patients had demonstrated non-oropharyngeal HPV+ primary tumors, both having tumors arising in the nasopharynx.55-57
In the KEYNOTE-012 expansion cohort, 132 patients with r/m HNSCC, regardless of PD-L1 status, received a fixed dose of pembrolizumab 200 mg once every 3 weeks.58 ORR was again 18% (95% CI, 12%-26%); there was a statistically significant superior ORR for PD- L1+ versus PD-L1– patients (22% vs 4%; P = .021). Median duration of response was not reached in this cohort, and 6-month progression-free survival (PFS) and OS rates were 23% and 59%, respectively. Based on these data, pembrolizumab was approved by the FDA on August 5, 2016, for the treatment of patients with r/m HNSCC with disease progression on or after platinum-containing chemotherapy.45
Nivolumab is a fully human immunoglobulin G4 (IgG4) anti–PD-1 monoclonal antibody. It was studied in a phase III trial (CheckMate 141) among patients with platinum-refractory HNSCC.59 The definition of platinum-refractory included patients with tumor progression or recurrence within 6 months after platinum-based chemotherapy, which could have been administered in the context of primary, adjuvant, or r/m disease. Patients were randomly assigned in a 2:1 ratio to intravenous nivolumab 3 mg/ kg every 2 weeks or a standard investigator-chosen single-agent therapy of methotrexate, docetaxel, or cetuximab. The primary endpoint was OS and main secondary endpoints were PFS, ORR, and quality-of-life (QOL) assessments. Two hundred forty patients received nivolumab and 121 received standard therapy, with median OS of 7.5 months (95% CI, 5.5-9.1 months) in the nivolumab group versus 5.1 months (95% CI, 4-6 months) in the standard-therapy group. There was no significant difference in PFS, with median PFS 2.0 months (95% CI, 1.9-2.1 months) versus 2.3 months (95% CI, 1.9-3.1 months) in the nivolumab and standard-therapy groups, respectively. However, PFS at 6 months was improved with nivolumab therapy at 19.7% versus 9.9% (P = .32). The response rate (RR) was 13.3% in the nivolumab-treated patients versus 5.8% in the standard-therapy group. The nivolumab group experienced fewer grade 3 or 4 drug-related AEs, with the most frequent AEs being fatigue, nausea, rash, decreased appetite, and pruritus. Additionally, patients treated with nivolumab experienced less decline in patient-reported QOL compared with those receiving standard therapy. Patients with PD-L1 expression of >1% (OS 8.7 vs 4.6 months for PD-L1 <1%) and p16+ tumors (OS 9.1 vs 4.4 months for p16– tumors) had a greater response to nivolumab than to standard therapy; OS was not significantly different between PD-L1– patients treated with nivolumab or standard of care (HR, 0.89; 5.7 vs 5.8 months). Nivolumab appeared beneficial no matter which standard-of-care agent was given, and was beneficial both in patients whose prior cisplatin exposure had been in the primary therapy setting as well as in those who had received cisplatin for r/m disease. Nivolumab was approved by the FDA on November 10, 2016, for the treatment of patients with r/m HNSCC with disease progression on or after a platinum-based therapy.44
Durvalumab is an IgG1 monoclonal antibody to PD-L1. It has shown activity in a phase I/II multicenter open-label study (NCT01693562) as monotherapy for multiple solid tumor types, including HNSCC.60,61 Fifty-one patients with HNSCC were evaluated for response; ORR was 12% (25% in PD-L1+ patients); and disease control at 24 weeks was 16% (25% in PD-L1+ patients). Ongoing trials are assessing durvalumab as monotherapy or in combination with the anti–CTLA-4 antibody tremelimumab in the first- and second-line recurrent or metastatic setting (Table 1).
Atezolizumab and avelumab are both humanized mAbs against PD-L1 that have been tested across all tumor types and show an acceptable safety profile.62 Avelumab is being used in combination with standard-of-care chemoradiation in locally advanced disease in a phase III trial (NCT02952586).
CTLA-4 Inhibitors
Ipilimumab is a fully humanized monoclonal antibody that targets CTLA-4. It is being used with cetuximab and intensity-modulated radiotherapy (RT) in the frontline setting to treat locally advanced HNSCC (NCT01935921). Tremelimumab (MedImmune) is a selective human IgG2 mAb that is also an inhibitor of CTLA-4.63
Biomarkers of Interest
It has been shown that PD-L1 expression correlates to increased RRs to anti–PD-1 inhibitors.64,65 Challenges to the use of PD-L1 as a biomarker include fluctuation in expression at different time points, variation within tumor tissue,66-68 lack of uniformity in cutpoints employed in different trials, and multiple assays that introduce variability (and ultimately misclassification) in staining patterns.69,70 In HNSCC, at least 1 study has demonstrated that PD-L1 expression is a favorable prognostic feature only when present on tumor-infiltrating immune cells rather than on tumor cells.71 PD-L2 staining on tumor and inflammatory cells is associated with higher ORR (23% vs 10%), as compared with PD-L2–negative.72 Levels of peripheral blood CD8+ T lymphocytes both at baseline and during treatment were higher in those patients who responded to nivolumab.73 Responsiveness has also been linked to inflamed phenotype, as evidenced by an IFN-gamma–response gene signature.74 In other malignancies, TILs75,76 and mutational load77,78 are associated with response to immune checkpoint inhibition. As noted above, a moderate to high mutational load79 is present in both HPV– positive and HPV–negative HNSCC.
Rationale for Checkpoint Inhibitor Combinations
The activity of PD-1 inhibition in platinum-refractory HNSCC has led to interest in incorporating immune checkpoint inhibition into earlier lines of therapy, particularly in combination with conventional treatments. A significant body of evidence provides rationale for this strategy.
By increasing the presentation of tumor antigens, promoting immunogenic cell death, and influencing the tumor microenvironment, cytotoxic chemotherapy creates a favorable environment for synergy with checkpoint inhibitors. In mouse models, cyclophosphamide has been shown to deplete Tregs and limit their suppressive capability.80,81 Myeloid-derived suppressor cells (MDSCs) aid in immune tolerance to cancer by inhibiting CD8+ T cells or cytotoxic T lymphocytes (CTLs). They are selectively killed by 5-fluorouracil (5-FU), causing CD8+ T-cell infiltration and antitumor responses both in vivo and in vitro.82 Similarly, gemcitabine also selectively targets MDSC, and, in combination with IFN-beta immunotherapy, increases antitumor effect.83 Certain chemotherapies increase expression of NK cell group 2D ligands, which are activating receptors involved in immunosurveillance expressed on NK cells and CTLs, causing an increase in tumor-cell lysis.84,85 Anthracyclines and oxaliplatin cause immunogenic cell death86 via 2 mechanisms. The first is an increased engulfment of tumor cells by DCs,87-89 while the other is by triggering a release of high-mobility group box 1 protein from dying tumor cells that acts on toll-like receptor 4 (TLR-4) expressed by DCs, optimizing the presentation and processing of tumor antigens.90 Cisplatin has been shown to broaden the range of tumor antigens exposed to CTL responses in vivo.91 Additionally, in mouse models it sensitizes tumor cells to CTL-mediated attacks through upregulation of mannose-6-phosphate receptors.92 In breast cancer, neoadjuvant anthracycline-based chemotherapy has been shown to change the immune infiltrate of the tumor93; those with an increase in tumor-infiltrating CTLs and a decrease in Tregs have significantly better rates of pathologic complete response (CR) at time of surgery.94
The method by which chemotherapy is administered can cause varying effects on the immune system. Dose-dense chemotherapy, a more frequent condensed administration of doses, has been shown to have improved disease-free survival (DFS) as adjuvant treatment for breast cancer95 and improved PFS for advanced ovarian cancer.96 In patients with ovarian cancer, dose-dense cisplatin + paclitaxel causes increase in CD8+ cytotoxic T cells, and this regimen decreases MDSCs as well as Tregs.97 Multiple chemotherapeutic agents have been shown to induce PD-L1 expression in tumor cells,98 and this upregulation has been associated with worse outcomes.99 This is another rationale for combination, with anti–PD-1/PD-L1 drugs, which have better response rates in this setting.
The combination of cytotoxic chemotherapy with checkpoint inhibitors can potentially provide more rapid disease control while waiting for immunotherapy response (the median time to response with pembrolizumab monotherapy was 8 weeks48) and reduce tumor size to allow for better T-cell infiltration. Multiple trials in non–small-cell lung cancer (NSCLC) have tested anti–PD-1 anti- bodies with chemotherapy. The KEYNOTE-021 study100 compared pembrolizumab plus carboplatin/pemetrexed with chemotherapy alone, while the CheckMate 012 trial101 compared nivolumab plus various combinations of platinum-doublets with nivolumab monotherapy and with chemotherapy alone. Combination chemo- therapy/PD-1 agent arms in both trials had higher ORRs of 55% and 33% to 47% (across arms), respectively, as compared with the historical rates with PD-1 treatment alone (44.8%102 and 19%,103 respectively). Analogously in HNSCC, pembrolizumab is being combined with platinum/5-FU/cetuximab in the first-line r/m setting and with docetaxel in the second-line setting. Similarly, nivolumab is being combined with cisplatin/radiation in the frontline locally advanced setting.
The use of combination immunotherapy, or anti–PD-1/anti–PD-L1 and anti–CTLA-4 antibodies, may hold promise in HNSCC through a synergistic effect. They bind to their ligands at different points in T-cell development104 and have shown promise in preclinical models of HNSCC.105 In vivo, antibodies targeting the 2 pathways differ in their immune effects, with CTLA-4 blockade causing increase in memory T cells and PD-1 blockade causing alteration of genes responsible for T-cell and NK function.106 Clinically, phase I and II trials in melanoma and NSCLC107 showed significantly improved response rates with nivolumab/ipilimumab as compared with monotherapy. In the r/m setting, anti–CTLA-4 drugs have been combined with nivolumab (anti–PD-1) in many trials in other solid tumors. Thus, there is rationale for the ongoing phase I/II trials of nivolumab/ipilimumab and durvalumab/tremelimumab.
The targeted therapies act on a variety of molecular pathways, generally have a more rapid onset of action with shorter-lived benefit, and can work synergistically with immunomodulatory agents.108 Cetuximab, a monoclonal antibody to EGFR, has been shown in colon cancer cell lines (in combination with chemotherapy) to promote activation of hu- man DCs for antigen priming and to create a vigorous CTL response.109 Cetuximab acts via antibody-dependent cellular cytotoxicity through CD56+ NK cells in patients with metastatic colon cancer,110 but it has also been shown to act through complement-dependent cytotoxicity in lung cancer cell lines.111,112 Cetuximab induces immunosuppressive Tregs that express CTLA-4,113 and it is being combined with ipilimumab in a phase I trial (NCT01935921).
The transcription factor signal transducer and activation of transcription 3 (STAT3) functions through an immunosuppressive pathway in which activation promotes expansion of MDSCs and Tregs, and causes abnormal DC differentiation.114,115 JAK2/STAT3 pathway-selective inhibitors have been shown in mouse models to promote differentiation of mature DCs, increase T-cell priming, and have an antitumor effect.116 Combinations of pembrolizumab with a JAK1 inhibitor and durvalumab with a STAT3 inhibitor are undergoing investigation in early-phase trials.
Activation of the PI3K/Akt pathway causes immune resistance by suppression of an antiapoptotic pathway (suppresses Mcl-1), and tumor cell lines that express Akt are resistant to T-cell killing.117 Inhibition of the Akt pathway with targeted therapy increases CTL killing of tumor cells in vitro.118 Pembrolizumab is being combined with a PI3K-delta inhibitor (INCB050465) while nivolumab is added to a PI3K-gamma inhibitor (IPI-549) in phase I/II trials.
The histone deacetylase (HDAC) inhibitors deplete MDSCs, and in mouse models of metastatic cancer, they have been shown to be highly effective in combination with anti–PD-1/anti–CTLA-4 antibodies, completely curing metastatic disease.119 Another HDAC inhibitor, vorinostat, has been used in mouse models of melanoma, in which the mechanism of action is inhibition of the Fas/FasL-dependent activation-induced death of T cells. In combination with anti–CTLA-4 antibody, there was a synergistic antitumor effect.120 Vorinostat with pembrolizumab is currently enrolling patients in phase I testing.
Indoleamine 2,3-dioxygenase 1 (IDO1) is an enzyme that breaks down tryptophan, and when produced by activated DCs, it causes an inhibition of T-cell proliferation.121 Epacadostat, an oral selective inhibitor of IDO1, has had promising RRs in multiple solid tumor types (primarily advanced melanoma) in combination with pembrolizumab in a phase I study.122 There were 2 patients with HNSCC patients on the trial, both of whom had responses (1 partial, 1 stable disease). Epacadostat is being studied in the r/m HNSCC setting in combination with pembrolizumab and nivolumab.
5-azacitidine (Aza) is a DNA hypomethylating agent that is incorporated into DNA and blocks DNA methyltransferases. In NSCLC cell lines, it increases IFN signaling, which leads to upregulation of surface antigens and PD-L1.123,124 Another important pathway of Aza-related immune regulation is through cytosolic sensing of double-stranded RNA, releasing a type I IFN response that upregulates hypermethylated endogenous retrovirus genes.125 High expression of these “viral defense” genes correlates strongly with clinical benefit in ovarian cancer patients who have been treated with checkpoint inhibitor.126 In a mouse melanoma model, low- dose Aza enhanced the effect of anti–CTLA-4 therapy.125 Currently, Aza is being used in combination with durvalumab in early clinical trials.
RT also affects the immune system in ways that may be associated with immune exhaustion or activation. In a mouse model of colon cancer, RT increased new peptide formation, cell surface expression of major histocompatibility class (MHC) class I molecules, antigen presentation, and CTL recognition of irradiated cells.127 In another mouse model of melanoma, RT increased migration of antigen-presenting cells (APCs) to the tumor site, increased tumor-infiltrating lymphocytes (TILs) that secreted IFN-gamma, and increased circulating tumor antigens.128 The “abscopal effect” is a term that describes local RT causing tumor shrinkage at distant, nonirradiated sites, presumably through activation of the immune system.129,130 RT by itself has been shown to cause increased PD-L1 expression in mouse models.131 In a breast cancer mouse model, RT alone caused an increase in PD-L1 expression in tumor cells. When anti–PD-L1 antibody was given with RT, tumor growth was controlled, there was a decrease in MDSCs, and an abscopal effect was also demonstrated.132 In clinical case reports and small retrospective series, there has been evidence for the abscopal effect in NSCLC133 and melanoma.134 Specifically, in HNSCC, there are cell-line models that show chemotherapy with radiation enhances CTL killing and sensitization of HNSCC cells to the granule perforin/ granzyme pathway of CTL killing, while downregulating bcl-2 (antiapoptotic gene) expression.135 In another metastatic breast cancer mouse model, local radiation with anti–CTLA-4 blockade showed increased improvement in metastatic burden.136 Based on this justification, phase I trials combining checkpoint inhibitors with RT in the upfront setting are ongoing. The optimal timing of the combination, management of immune AEs without incurring treatment interruptions, and the possibility of increased toxicity are all unanswered questions in this arena. A phase II trial at Yale Cancer Center that may provide guidance on scheduling is currently enrolling patients with residual disease following chemoradiation to receive 3 months of pembrolizumab therapy prior to definitive resection (NCT02892201). Correlative studies will permit characterization of the postradiation tumor-immune microenvironment in relation to pembrolizumab response.
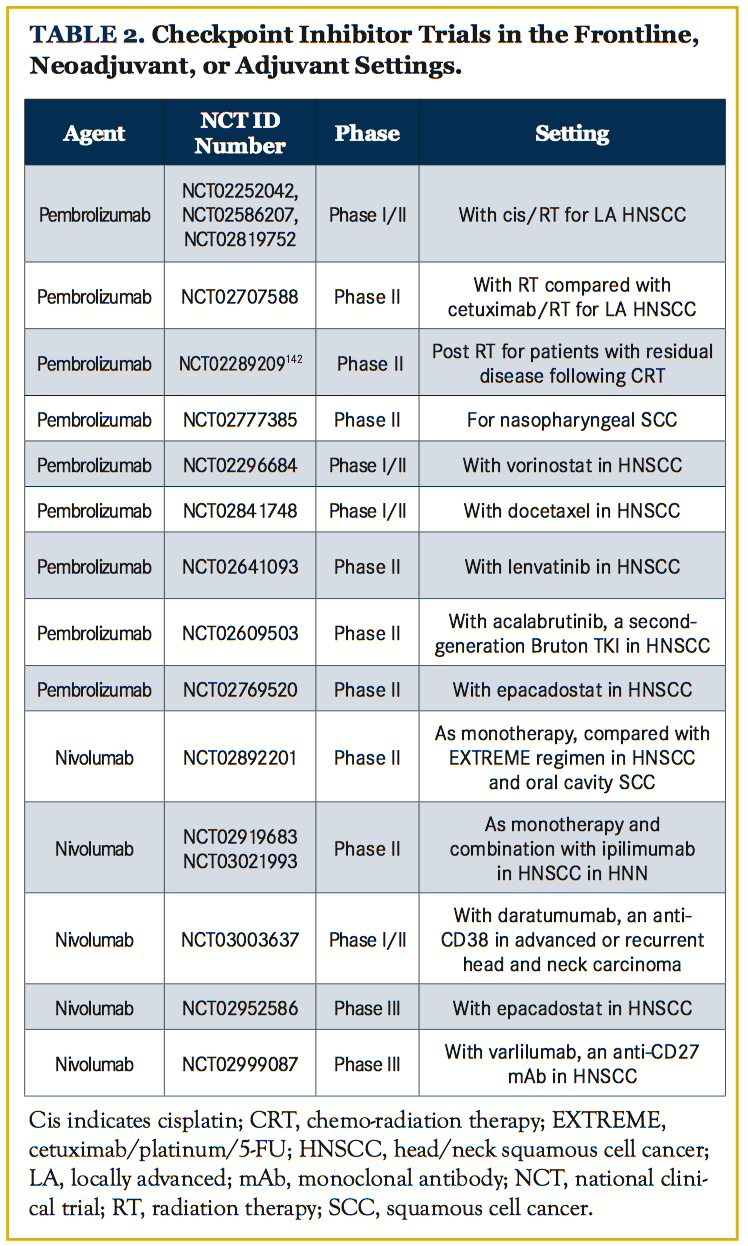
Surgery to remove primary tumor in metastatic breast cancer mouse models has been shown to reverse tumor-mediated immune suppression.137 This suggests that immune therapy in the adjuvant therapy setting may be more effective. The patients with HNSCC patients who should be targeted in the adjuvant setting are HPV– patients with locally advanced disease. Even with adjuvant cisplatin/ RT, 3-year disease-free survival rates for this group are currently 30% to 50%138-140, with an absolute benefit of cisplatin/RT of 6.5%.141,142 Ongoing trials of checkpoint inhibitors in the adjuvant setting with chemotherapy/RT are listed in Table 2.
Combinations of checkpoint inhibitors with vaccines, oncolytic tumor virus, and as monotherapy in the no-evidence-of-disease setting are also under investigation. The discovery of the means to augment the antitumor immune response in patients with HNSCC, and the recognition that a subset of patients treated with checkpoint inhibitors approach can expect long durations of disease control, are important breakthroughs in management of this difficult disease. Much remains to be learned, however, regarding patient selection, manipulation of the tumor microenvironment to enhance sensitivity to this approach, and the optimal means to integrate immunotherapy with definitive management to have the greatest impact on cure and on function preservation in patients with HNSCC.
Other Immunomodulators
Other inhibitory receptors control immune response. Among them is killer cell immunoglobulin-like receptor (KIR), which interacts with MHC-I molecules to suppress cytotoxicity.143 Anti-KIR antibodies may remove inhibitory signals on NK cells. Two phase I trials are testing an anti-KIR antibody (lirilumab) with nivolumab (NCT01714739) and with ipilimumab (NCT01750580).
Lymphocyte activation gene-3 (LAG-3) is an immune checkpoint protein that negatively regulates T cells and immune response by binding to MHC class II molecules.144,145 It has been found that LAG- 3 is overexpressed on TILs, and that overexpression correlates with higher pathologic grades, larger tumor size, and positive lymph node status in HNSCC.146 IMP 321, a soluble LAG-3 IgG fusion protein and MHC class II agonist, has shown clinical activity in phase I trials for metastatic pancreatic, breast, and renal cell cancers.147-149 BMS- 986016, an anti–LAG-3 antibody, is being used in phase I trials with nivolumab in HPV+ HNSCC (NCT02488759) as well as in advanced solid tumors including HPV+/– HNSCC (NCT01968109).
AMG228 is another Mab in phase 1 trials for HNSCC. It targets glucocorticoid-induced tumor necrosis factor (TNF)-related receptor, which is expressed on the surface of CD25+CD4+ regulatory T cells and is costimulatory toward effector T cells.150 It is being used in a phase I clinical trial across multiple tumor types (NCT02437916).
TIM-3, a molecule selectively expressed on helper T cells, has been shown to be a negative regulator of T cells.151 Elevated expression of TIM-3 has been shown in patients with HNSCC to be correlated with worse clinical outcomes.28 Anti–TIM-3 antibodies (TSR-022) are in phase I clinical trials across tumor types as mono- therapy (NCT02817633), with anti–PD-1 (NCT02608268) and with anti-TGF beta antibodies (NCT02947165).
Monalizumab (IPH2201) is a mAb-targeting NK cell lectin receptor (CD159) that is being tested in a phase I trial in combination with cetuximab in patients with r/m HNSCC; it is currently recruiting (NCT02643550).
Costimulatory Agonists
Toll-like receptors (TLRs)
Another immune therapy strategy is to enhance positive stimulatory pathways through cytokines and mAbs.152 The TLRs are a family of pathogen recognition receptors; the engagement of specific agonists in- duces activation of DCs and induces NK-cell–dependent lysis of tumor cells.153-155 TLR-8 activates monocytes, NK cells, and DCs, and can potentially have a synergistic effect with chemotherapy or Mab treatment.156 TLR8 agonist VTX-2337 has been tested with cetuximab in a phase I trial (NCT01334177), with preliminary data showing an acceptable safety profile in 10 patients with an overall disease control rate of 60%157; it was also being tested with the EXTREME regimen in a phase II trial (NCT01836029) in r/m HNSCC.
Motolimod, which showed no improvement in outcome with the addition of VTX-2337, another TLR-8 agonist, is being assessed with nivolumab in the neoadjuvant setting in a phase I trial (NCT02124850). EMD1201081, a TLR9 agonist, was added to cetuximab in r/m HNSCC in a phase II trial (NCT01040832); however, early results were not promising and were without clinical efficacy,158 and for those reasons the study was terminated. Additionally, there is a phase I/II study of durvalumab/tremelimumab in combination with tumor microenvironment modulatory poly-ICLC, a TLR-3 agonist, in advanced, measurable, biopsy-accessible HNSCC (NCT02643303).
OX40
OX40 is a TNF receptor family member that can enhance T-cell memory, proliferation, and antitumor activity. It has been found in abundance in the TILs of patients with advanced HNSCC.159 A phase I study is ongoing of MEDI6469, a mAb to OX40, in the neoadjuvant setting before surgical resection of locally advanced HNSCC (NCT02274155). In the r/m setting, it is being used as monotherapy or in combination with 4-1BB agonist PF-05082566, utomilumab (NCT02315066), and in combination with durvalumab or tremelimumab (NCT02205333).
CD137 (4-1BB)
Urelumab is a humanized mAb agonist of CD137, a TNF family receptor primarily expressed on activated T cells, DCs, and activated NK cells.160 An early phase I/II study reported in 2016 showed an ORR of 50% in patients with melanoma but only about 5% in the HNSCC cohort (n = 22) with the combination of urelumab and nivolumab.161 A phase Ib trial is ongoing, combining urelumab with cetuximab (NCT02110082) and with nivolumab (NCT02253992) in the r/m setting.
Another CD137 antibody is PF-05082566 (IgG2), or utomilumab. It is undergoing investigation in several phase I/II trials including with OX40 antibody (NCT02315066), with mogamulizumab (NCT02444793), with PD-1 inhibitor (NCT02179918; KEYNOTE 0036 [completed]), with PD-L1 inhibitor (NCT02554812), and as a single agent (NCT01307267).
CD40L
CD40L is expressed on activated CD4 T cells, and it binds to CD40 on APCs to activate them and to prime CD8 T cells.162 It is thought that this costimulatory pathway is downregulated in HNSCC immune escape.163,164 CP-870,893 is an anti-CD40 mAb that is being tested across multiple tumor types, including HNSCC, in a phase I trial as a monotherapy (NCT02225002). It has shown safety and efficacy across tumor types in phase I studies as monotherapy165 and in combination with chemotherapy.166
Viruses
Several therapeutic vaccines have shown results in phase I/II trials of HNSCC. Peptide immunomodulatory vaccines GL-0810 (HPV16) and GL-0817 (melanoma antigen E-A3 tumor-associated antigen, or MAGE-A3, a cancer-testis antigen) were developed as 2 separate vaccines using HLA-I and HLA-II T-cell epitopes of HPV16 and MAGE-A3 tumor-associated antigens. A phase I trial showed them to be well tolerated and that they demonstrated anti- body responses in the majority of patients (80% of the HPV16 cohort and 67% of the MAGE-A3 cohort).167 Cancer-testis antigens are good targets for peptide vaccines because they are specifically overexpressed on cancer cells as compared with normal tissue.
Another phase II trial with 37 patients looked at a peptide vaccine derived from 3 such antigens and showed increased CD8+ T-cell infiltration after vaccine use and that postvaccination peptide- specific CTL frequency was associated with OS; in addition, 1 patient demonstrated a CR.168
A phase I trial tested adjuvant peptide-loaded DC-based vaccination against p53 in 16 patients; the data showed that it was well-tolerated, decreased Tregs levels post vaccination, and resulted in a 2-year DFS of 88%.169 A current phase I trial is examining modified vaccinia virus Ankara vaccine expressing p53 in combination with pembrolizumab (NCT02432963) in the r/m setting.
Talimogene laherparepvec is an oncolytic herpes simplex virus 1 vaccine encoding granulocyte-macrophage colony-stimulating factor (GM- CSF) that has shown some promise in HNSCC; it was FDA-approved for the treatment of melanoma in 2015. It has been tested in a phase I/ II study with standard cisplatin/radiation for the first-line treatment of advanced stage III/IV HNSCC.170 At a median follow-up of 29 months, there was an OS of 70.5%; of note, patients had postoperative neck dissections, which is not the standard of care. It is currently in a phase I/ IIIb trial in combination with pembrolizumab in r/m HNSCC patients (NCT02626000) in the MASTERKEY232/KEYNOTE-137 trial.
JX-594, an oncolytic vaccinia virus with deletion of thymidine kinase and addition of GM-CSF, has an ongoing current phase I trial in the r/m setting (NCT00625456), with results still pending. Enadenotucirev is an oncolytic group B adenovirus that is being used in combination with nivolumab in a phase I trial (NCT02636036).
INO-3112 is a DNA vaccine that combines 2 previously developed DNA vaccines (plasmids encoding HPV16 and HPV18 E6/E7) that results in an HPV-specific CD8+ T cell response. In a prospective phase I/IIa trial in adults with HPV+ HNSCC treated definitively with either chemoradiation or surgery, the vaccine was well-tolerated and HPV-spe- cific T-cell immunity was generated by antibody titers.171
The Hespecta vaccine family (the acronym is derived from HPV E Six Peptide Conjugated To Amplivant) includes ISA101 and ISA201, which are peptide vaccines derived from HPV16 E6 and E7 proteins. ISA101 has given promising results in patients with vulvar intraepithelial neoplasia,172,173 but no results are yet in from HNSCC patients. ISA101 is being used in monotherapy and in combination with nivolumab in a phase II trial (NCT02426892) for HPV16+ tumors. ISA201 is a second-generation vaccine in which 2 HPV16 E6 peptides are conjugated to a TLR2 agonist. A phase I trial (NCT02821494) of ISA201 is ongoing for HPV+ tumors that were definitively treated.
Conclusions
Novel immunotherapies have shown promising initial results in HNSCC. The checkpoint inhibitors pembrolizumab and nivolumab are now established in the treatment paradigm for metastatic dis- ease. Moving forward, the results of many early-phase clinical trials should help guide the use of checkpoint inhibitors in the frontline, neoadjuvant, and adjuvant settings. In the future, combinations of immunotherapies and novel drugs may also replace monotherapy in r/m disease. We continue to wait eagerly for data from the phase I/II trials of vaccines and costimulatory agonists to see if they will become part of the treatment algorithm for HNSCC.
Affiliations: Tejas Suresh, MD, and Barbara A. Burtness, MD, are both physicians in the Department of Medical Oncology, Yale School of Medicine, New Haven, Connecticut.
Address correspondence to: Tejas Suresh, MD, Yale University School of Medicine, Department of Medical Oncology, 333 Cedar Street, WWW 221, PO Box 208028, New Haven, CT 06520-8028.
E-mail: [email protected].
Financial disclosures: None

References
- Global Burden of Disease Collaboration; Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524-548. doi: 10.1001/jamaoncol.2016.5688.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387.
- Rampias T, Sasaki C, Weinberger P, Psyrri A. E6 and e7 gene silencing and transformed phenotype of human papillomavirus 16-positive oropharyngeal cancer cells. J Natl Cancer Inst. 2009;101(6):412-423. doi: 10.1093/jnci/djp017.
- Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24(5):736-747.
- O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440-451. doi: 10.1016/S1470-2045(15)00560-4.
- Westra WH. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol. 2009;3(1):78-81. doi: 10.1007/s12105-009-0100-y.
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemo- therapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116-1127. doi: 10.1056/NEJMoa0802656.
- Argiris A, Li S, Ghebremichael M, et al. Prognostic significance of human papillomavirus in recurrent or metastatic head and neck cancer: an analysis of Eastern Cooperative Oncology Group trials. Ann Oncol. 2014;25(7):1410-1416. doi: 10.1093/annonc/mdu167.
- Roberts SA, Lawrence MS, Klimczak LJ, et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat Genet. 2013;45(9):970-976. doi: 10.1038/ng.2702.
- Keck MK, Zuo Z, Khattri A, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21(4):870-881. doi: 10.1158/1078-0432. CCR-14-2481.
- Seiwert TY, Zuo Z, Keck MK, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21(3):632-641. doi: 10.1158/1078-0432.CCR-13-3310.
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576-582. doi: 10.1038/nature14129.
- Allen CT, Judd NP, Bui JD, Uppaluri R. The clinical implications of antitumor immunity in head and neck cancer. Laryngoscope. 2012;122(1):144-157. doi: 10.1002/lary.21913.
- Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92(5):913-920.
- Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13(15 Pt 1):4345-4354.
- Duray A, Demoulin S, Hubert P, Delvenne P, Saussez S. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010;2010:701657. doi: 10.1155/2010/701657.
- Bose A, Chakraborty T, Chakraborty K, Pal S, Baral R. Dysregulation in immune functions is reflected in tumor cell cytotoxicity by peripheral blood mononuclear cells from head and neck squamous cell carcinoma patients. Cancer Immun. 2008;8:10.
- Brocks CP, Pries R, Frenzel H, Ernst M, Schlenke P, Wollenberg B. Functional alteration of myeloid dendritic cells through head and neck cancer. Anticancer Res. 2007;27(2):817-824.
- Molling JW, Langius JA, Langendijk JA, et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25(7):862-868.
- Echarri MJ, Lopez-Martin A, Hitt R. Targeted therapy in locally advanced and recurrent/metastatic head and neck squamous cell carcinoma (LA-R/M HNSCC). Cancers (Basel). 2016;8(3). pii: E27. doi: 10.3390/cancers8030027.
- Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423-449. doi:10.1146/annurev.immunol.18.1.423.
- Fontenot JD, Gavin MA, Rudensky AY. Pillars article: Foxp3 pro- grams the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003: 4: 330-336. J Immunol. 2017;198(3):986-992.
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057-1061.
- Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9(2):606-612.
- Woo EY, Yeh H, Chu CS, et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168(9):4272-4276.
- Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(18):5423-5434.
- Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9(12):4404-4408.
- Jie HB, Gildener-Leapman N, Li J, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109.
- Ghiringhelli F, Menard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. The Journal of experimental medicine. 2005;202(8):1075-1085.
- Ralainirina N, Poli A, Michel T, et al. Control of NK cell functions by CD4+CD25+ regulatory T cells. Journal of leukocyte biology. 2007;81(1):144-153.
- Albers AE, Ferris RL, Kim GG, Chikamatsu K, DeLeo AB, Whiteside TL. Immune responses to p53 in patients with cancer: enrichment in tetramer+ p53 peptide-specific T cells and regulatory T cells at tumor sites. Cancer immunology, immunotherapy : CII. 2005;54(11):1072-1081.
- Jie HB, Schuler PJ, Lee SC, et al. CTLA-4(+) Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015;75(11):2200-2210.
- Ferris RL. Immunology and Immunotherapy of Head and Neck Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(29):3293-3304.
- Kammertoens T, Schüler T, Blankenstein T. Immunotherapy: target the stroma to hit the tumor. Trends in Molecular Medicine. 2005;11(5):225-231.
- Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(21):6301-6311.
- Brahmer JR. Harnessing the immune system for the treatment of non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(8):1021-1028.
- Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(24):6580-6587.
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111-122.
- Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nature reviews. Immunology. 2011;11(12):852-863.
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. International immunology. 2007;19(7):813-824.
- Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. The New England journal of medicine. 2016;375(18):1767-1778.
- Concha-Benavente F, Srivastava RM, Trivedi S, et al. Identification of the Cell-Intrinsic and -Extrinsic Pathways Downstream of EGFR and IFNgamma That Induce PD-L1 Expression in Head and Neck Cancer. Cancer Res. 2016;76(5):1031-1043.
- Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73(1):128-138.
- Administration USFaD. OPDIVO (nivolumab) full prescribing information2016.
- Administration USFaD. Pembrolizumab approval2016.
- Administration USFaD. Tecentriq prescribing information. 2016.
- Administration USFaD. Yervoy Prescribing Information 2011.
- Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. The Lancet. Oncology. 2016;17(7):956-965.
- Robert C, Hodi FS, Wolchok JD, et al. Association of response to programmed death receptor 1 (PD-1) blockade with pembrolizumab (MK-3475) with an interferon-inflammatory immune gene signature. Journal of Clinical Oncology. 2015;33(15_suppl):3001-3001.
- Machiels J-PH, Coulie PG. The promise of immunostimulatory antibodies in head and neck cancer. The Lancet Oncology.17(7):856-857.
- Hitt R, Amador ML, Quintela-Fandino M, et al. Weekly docetaxel in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Cancer. 2006;106(1):106-111.
- Khanna R, Busson P, Burrows SR, et al. Molecular characterization of antigen-processing function in nasopharyngeal carcinoma (NPC): evidence for efficient presentation of Epstein-Barr virus cytotoxic T-cell epitopes by NPC cells. Cancer Res. 1998;58(2):310-314.
- Pai S, O'Sullivan B, Abdul-Jabbar I, et al. Nasopharyngeal carcinoma-associated Epstein-Barr virus-encoded oncogene latent membrane protein 1 potentiates regulatory T-cell function. Immunology and cell biology. 2007;85(5):370-377.
- Zhou Y, Shi D, Miao J, et al. PD-L1 predicts poor prognosis for nasopharyngeal carcinoma irrespective of PD-1 and EBV-DNA load. Scientific reports. 2017;7:43627.
- Combes J-D, Franceschi S. Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral oncology. 2014;50(5):370-379.
- Schlecht NF, Brandwein-Gensler M, Nuovo GJ, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24(10):1295-1305.
- Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ. Significance of p16 in Site-specific HPV Positive and HPV Negative Head and Neck Squamous Cell Carcinoma. Cancer and clinical oncology. 2013;2(1):51-61.
- Chow LQ, Haddad R, Gupta S, et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016.
- Ferris RL, Blumenschein G, Jr., Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. The New England journal of medicine. 2016;375(19):1856-1867.
- Segal NHea. A phase I multi-arm dose-expansion study of the anti-programmed cell death-ligand 1 (PD-L1) antibody MEDI4736: Preliminary Data. presented at: ESMO; 9/28/2014, 2014.
- Segal NHea. Safety and effiacy of MEDI4736, an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. presented at: ASCO Annual Meeting2015.
- Patel MR, Infante JR, Iannotti N, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with metastatic or locally advanced solid tumors: assessment of safety and tolerability in a phase I, open-label expansion study. Journal of Clinical Oncology. 2015;33(15_suppl):3044-3044.
- Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(35):8968-8977.
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;372(21):2018-2028.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. New England Journal of Medicine. 2012;366(26):2443-2454.
- M. K. Evaluation of PD-L1 expression in metachronous tumor samples and FDG-PET as a predictive biomarkers in Ph2 study of Atezolizumab. presented at: World Conference on Lung Cancer2015; Denver.
- N. D. Spatiotemporal effects on Programmed Death Ligand 1 (PD-L1) expression and Immunophenotype of Non-small Cell Lung Cancer (NSCLC). presented at: World Conference on Lung Cancer 2015; Denver.
- Sheffield BS GG, Pleasance E. Predictive biomarker testing for programmed cell death 1 inhibition in Non-small lung cancer presented at: World Conference on Lung Cancer 2015; Denver.
- Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Laboratory investigation; a journal of technical methods and pathology. 2014;94(1):107-116.
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2017;12(2):208-222.
- Kim HR, Ha SJ, Hong MH, et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Scientific reports. 2016;6:36956.
- Chow LQea. Biomarkers and response to pembrolizumab (pembro) in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). presented at: ASCO2016.
- Ferris Rea. Characterization of potential predictive biomarkers of response to nivolumab in CheckMate-141 in patients with squamous cell carcinoma of the head and neck (SCCHN). presented at: ASCO-SITC Clinical Immuno-Oncology Symposium2017.
- al TYSe. Inflamed-phenotype gene expression signatures to predict benefit from the anti-PD-1 antibody pembrolizumab in PD-L1+ head and neck cancer patients presented at: 2015 ASCO Annual Meeting 2015.
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568-571.
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. New England Journal of Medicine. 2015;372(26):2509-2520.
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124-128.
- Snyder A, Makarov V, Merghoub T, et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. New England Journal of Medicine. 2014;371(23):2189-2199.
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415-421.
- Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. European journal of immunology. 2004;34(2):336-344.
- Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105(7):2862-2868.
- Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052-3061.
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(18):6713-6721.
- Armeanu S, Bitzer M, Lauer UM, et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65(14):6321-6329.
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186-1190.
- Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? The Journal of Clinical Investigation. 2008;118(6):1991-2001.
- Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. The Journal of experimental medicine. 2005;202(12):1691-1701.
- Obeid M, Panaretakis T, Joza N, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell death and differentiation. 2007;14(10):1848-1850.
- Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54-61.
- Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050-1059.
- Jackaman C, Majewski D, Fox SA, Nowak AK, Nelson DJ. Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8(+) T cells in vivo. Cancer immunology, immunotherapy : CII. 2012;61(12):2343-2356.
- Ramakrishnan R, Assudani D, Nagaraj S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120(4):1111-1124.
- Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109(8):2796-2801.
- Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(1):105-113.
- Bonilla L, Ben-Aharon I, Vidal L, Gafter-Gvili A, Leibovici L, Stemmer SM. Dose-dense chemotherapy in nonmetastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. J Natl Cancer Inst. 2010;102(24):1845-1854.
- Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet (London, England). 2009;374(9698):1331-1338.
- Chang C-L, Hsu Y-T, Wu C-C, et al. Dose-Dense Chemotherapy Improves Mechanisms of Antitumor Immune Response. Cancer Research. 2013;73(1):119-127.
- Peng J, Hamanishi J, Matsumura N, et al. Chemotherapy Induces Programmed Cell Death-Ligand 1 Overexpression via the Nuclear Factor-kappaB to Foster an Immunosuppressive Tumor Microenvironment in Ovarian Cancer. Cancer Res. 2015;75(23):5034-5045.
- Zhang P, Ma Y, Lv C, et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer science. 2016;107(11):1563-1571.
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. The Lancet Oncology.17(11):1497-1508.
- Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in Combination With Platinum‐Based Doublet Chemotherapy for First-Line Treatment of Advanced Non–Small-Cell Lung Cancer. Journal of Clinical Oncology. 2016;34(25):2969-2979.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2016;375(19):1823-1833.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;373(17):1627-1639.
- Economopoulou P, Agelaki S, Perisanidis C, Giotakis EI, Psyrri A. The promise of immunotherapy in head and neck squamous cell carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology. 2016;27(9):1675-1685.
- Swanson MS, Sinha UK. Rationale for combined blockade of PD-1 and CTLA-4 in advanced head and neck squamous cell cancer-review of current data. Oral oncology. 2015;51(1):12-15.
- Das R, Verma R, Sznol M, et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. Journal of immunology (Baltimore, Md. : 1950). 2015;194(3):950-959.
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. The Lancet Oncology. 2017;18(1):31-41.
- Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nature reviews. Cancer. 2012;12(4):237-251.
- Correale P, Botta C, Cusi MG, et al. Cetuximab +/- chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int J Cancer. 2012;130(7):1577-1589.
- Marechal R, De Schutter J, Nagy N, et al. Putative contribution of CD56 positive cells in cetuximab treatment efficacy in first-line metastatic colorectal cancer patients. BMC cancer. 2010;10:340.
- Dechant M, Weisner W, Berger S, et al. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res. 2008;68(13):4998-5003.
- Hsu YF, Ajona D, Corrales L, et al. Complement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivo. Molecular cancer. 2010;9:139.
- Srivastava RM, Lee SC, Andrade Filho PA, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(7):1858-1872.
- Lee H, Pal SK, Reckamp K, Figlin RA, Yu H. STAT3: A Target to Enhance Antitumor Immune Response. Current topics in microbiology and immunology. 2011;344:41-59.
- Nefedova Y, Cheng P, Gilkes D, et al. Activation of dendritic cells via inhibition of Jak2/STAT3 signaling. Journal of immunology (Baltimore, Md. : 1950). 2005;175(7):4338-4346.
- Nefedova Y, Nagaraj S, Rosenbauer A, Muro-Cacho C, Sebti SM, Gabrilovich DI. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 2005;65(20):9525-9535.
- Hahnel PS, Thaler S, Antunes E, Huber C, Theobald M, Schuler M. Targeting AKT signaling sensitizes cancer to cellular immunotherapy. Cancer Res. 2008;68(10):3899-3906.
- Noh KH, Kang TH, Kim JH, et al. Activation of Akt as a mechanism for tumor immune evasion. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(3):439-447.
- Kim K, Skora AD, Li Z, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A. 2014;111(32):11774-11779.
- Cao K, Wang G, Li W, et al. Histone deacetylase inhibitors prevent activation-induced cell death and promote anti-tumor immunity. Oncogene. 2015;34(49):5960-5970.
- Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. Journal of immunology (Baltimore, Md. : 1950). 2000;164(7):3596-3599.
- Gangadhar TC, Hamid O, Smith DC, et al. Epacadostat plus pembrolizumab in patients with advanced melanoma and select solid tumors: Updated phase 1 results from ECHO-202/KEYNOTE-037. Annals of Oncology. 2016;27(suppl_6):1110PD-1110PD.
- Li H, Chiappinelli KB, Guzzetta AA, et al. Immune regulation by low doses of the DNA methyltransferase inhibitor 5-azacitidine in common human epithelial cancers. Oncotarget. 2014;5(3):587-598.
- Wrangle J, Wang W, Koch A, et al. Alterations of immune response of Non-Small Cell Lung Cancer with Azacytidine. Oncotarget. 2013;4(11):2067-2079.
- Chiappinelli Katherine B, Strissel Pamela L, Desrichard A, et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell. 2015;162(5):974-986.
- Verhaak RG, Tamayo P, Yang JY, et al. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest. 2013;123(1):517-525.
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. The Journal of experimental medicine. 2006;203(5):1259-1271.
- Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local Radiation Therapy of B16 Melanoma Tumors Increases the Generation of Tumor Antigen-Specific Effector Cells That Traffic to the Tumor. The Journal of Immunology. 2005;174(12):7516-7523.
- Levy A, Chargari C, Cheminant M, Simon N, Bourgier C, Deutsch E. Radiation therapy and immunotherapy: implications for a combined cancer treatment. Critical reviews in oncology/hematology. 2013;85(3):278-287.
- Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer treatment reviews. 2015;41(6):503-510.
- Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2(9):831-838.
- Deng L, Liang H, Burnette B, et al. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. The Journal of Clinical Investigation. 2014;124(2):687-695.
- Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1(6):365-372.
- Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780.
- Gelbard A, Garnett CT, Abrams SI, et al. Combination Chemotherapy and Radiation of Human Squamous Cell Carcinoma of the Head and Neck Augments CTL-Mediated Lysis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(6):1897-1905.
- Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(2 Pt 1):728-734.
- Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64(6):2205-2211.
- Bernier J, Domenge C, Ozsahin M, et al. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. New England Journal of Medicine. 2004;350(19):1945-1952.
- Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. New England Journal of Medicine. 2004;350(19):1937-1944.
- Harari PM, Harris J, Kies MS, et al. Postoperative chemoradiotherapy and cetuximab for high-risk squamous cell carcinoma of the head and neck: Radiation Therapy Oncology Group RTOG-0234. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(23):2486-2495.
- Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2009;92(1):4-14.
- Husain Z. Pembrolizumab in HNSCC With Residual Disease After Radiation ClinicalTrials.gov; 2016.
- Campbell KS, Purdy AK. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology. 2011;132(3):315-325.
- Baixeras E, Huard B, Miossec C, et al. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. The Journal of experimental medicine. 1992;176(2):327-337.
- Triebel F, Jitsukawa S, Baixeras E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. The Journal of experimental medicine. 1990;171(5):1393-1405.
- Deng W-W, Mao L, Yu G-T, et al. LAG-3 confers poor prognosis and its blockade reshapes antitumor response in head and neck squamous cell carcinoma. OncoImmunology. 2016;5(11):e1239005.
- Brignone C, Escudier B, Grygar C, Marcu M, Triebel F. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(19):6225-6231.
- Brignone C, Gutierrez M, Mefti F, et al. First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. Journal of translational medicine. 2010;8:71.
- Wang-Gillam A, Plambeck-Suess S, Goedegebuure P, et al. A phase I study of IMP321 and gemcitabine as the front-line therapy in patients with advanced pancreatic adenocarcinoma. Investigational new drugs. 2013;31(3):707-713.
- Nocentini G, Ronchetti S, Petrillo MG, Riccardi C. Pharmacological modulation of GITRL/GITR system: therapeutic perspectives. British journal of pharmacology. 2012;165(7):2089-2099.
- Hastings WD, Anderson DE, Kassam N, et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. European journal of immunology. 2009;39(9):2492-2501.
- al SNe. Immunotherapy and Checkpoint Inhibitors in Recurrent and Metastatic Head and Neck Cancer: ASCO; 2016.
- Ghosh TK, Mickelson DJ, Fink J, et al. Toll-like receptor (TLR) 2-9 agonists-induced cytokines and chemokines: I. Comparison with T cell receptor-induced responses. Cellular immunology. 2006;243(1):48-57.
- Gorden KB, Gorski KS, Gibson SJ, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. Journal of immunology (Baltimore, Md. : 1950). 2005;174(3):1259-1268.
- Schreibelt G, Tel J, Sliepen KH, et al. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer immunology, immunotherapy : CII. 2010;59(10):1573-1582.
- Lu H, Dietsch GN, Matthews MA, et al. VTX-2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(2):499-509.
- Cohen EEW, Ferris RL, Gash K, et al. Active8: A randomized, double-blind, placebo-controlled study of chemotherapy plus cetuximab in combination with VTX-2337 in patients with recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN). Journal of Clinical Oncology. 2014;32(15_suppl):TPS3123-TPS3123.
- Ruzsa A, Sen M, Evans M, et al. Phase 2, open-label, 1:1 randomized controlled trial exploring the efficacy of EMD 1201081 in combination with cetuximab in second-line cetuximab-naive patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). Investigational new drugs. 2014;32(6):1278-1284.
- Bell RB, Leidner RS, Crittenden MR, et al. OX40 signaling in head and neck squamous cell carcinoma: Overcoming immunosuppression in the tumor microenvironment. Oral oncology. 2016;52:1-10.
- Lynch DH. The promise of 4-1BB (CD137)-mediated immunomodulation and the immunotherapy of cancer. Immunological reviews. 2008;222:277-286.
- al. MEe. Clinical safety and efficacy assessment of the CD137 agonist urelumab alone and in combination with nivolumab in patients with hematologic and solid tumor malignancies. presented at: SITC2016.
- Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(5):1035-1043.
- Davis RJ, Ferris RL, Schmitt NC. Costimulatory and coinhibitory immune checkpoint receptors in head and neck cancer: unleashing immune responses through therapeutic combinations. Cancers of the Head & Neck. 2016;1(1):12.
- Sathawane D, Kharat RS, Halder S, et al. Monocyte CD40 expression in head and neck squamous cell carcinoma (HNSCC). Human immunology. 2013;74(1):1-5.
- Johnson P, Challis R, Chowdhury F, et al. Clinical and biological effects of an agonist anti-CD40 antibody: a Cancer Research UK phase I study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(6):1321-1328.
- Vonderheide RH, Burg JM, Mick R, et al. Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. Oncoimmunology. 2013;2(1):e23033.
- Zandberg DP, Rollins S, Goloubeva O, et al. A phase I dose escalation trial of MAGE-A3- and HPV16-specific peptide immunomodulatory vaccines in patients with recurrent/metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN). Cancer Immunology, Immunotherapy. 2015;64(3):367-379.
- Yoshitake Y, Fukuma D, Yuno A, et al. Phase II Clinical Trial of Multiple Peptide Vaccination for Advanced Head and Neck Cancer Patients Revealed Induction of Immune Responses and Improved OS. Clinical Cancer Research. 2015;21(2):312-321.
- Schuler PJ, Harasymczuk M, Visus C, et al. Phase I Dendritic Cell p53 Peptide Vaccine for Head and Neck Cancer. Clinical Cancer Research. 2014;20(9):2433-2444.
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(15):4005-4015.
- Yang Z, Aggarwal C, Cohen R, et al. 1PDImmunotherapy with INO-3112 (HPV16 and HPV18 plasmids + IL-12 DNA) in human papillomavirus (HPV) associated head and neck squamous cell carcinoma (HNSCCa). Annals of Oncology. 2015;26(suppl_8):viii1-viii1.
- Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. The New England journal of medicine. 2009;361(19):1838-1847.
- van Poelgeest MI, Welters MJ, Vermeij R, et al. Vaccination against Oncoproteins of HPV16 for Noninvasive Vulvar/Vaginal Lesions: Lesion Clearance Is Related to the Strength of the T-Cell Response. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(10):2342-2350.





