Introduction
Major advances have occurred over the last decade for the treatment of metastatic non–small cell lung cancer (NSCLC), including both molecularly targeted therapies and immunotherapies. Treatment algorithms for metastatic disease are rapidly changing, providing patients with enhanced clinical benefit. However, despite targeted therapies leading to increased options for subsets of patients with nonsquamous NSCLC, the majority of patients with advanced NSCLC will not harbor a “targetable” genetic aberration in EGFR, anaplastic lymphoma kinase (ALK), or ROS1. Until very recently, in the first-line setting, platinum-based chemotherapy (with or without the addition of angiogenesis inhibitors in nonsquamous NSCLC) has been the mainstay of treatment for these patients.1
In rapid succession, 3 immune checkpoint inhibitors have been approved by the FDA since 2015 for the treatment of advanced patients with NSCLC who have progressed on standard platinum-based chemotherapy and for patients with EGFR mutations or ALK rearrangements who have also progressed on an FDA-approved targeted therapy. These are the programmed cell death protein 1 (PD-1) inhibitors nivolumab and pembrolizumab, and the programmed death ligand 1 (PD-L1) inhibitor atezolizumab.2-5 So far, pembrolizumab is the only immunotherapy that has received approval in the frontline setting, under 2 separate circumstances. The first approval was in October 2016 based on the randomized phase III KEYNOTE-024 study using pembrolizumab as monotherapy in 305 patients with NSCLC whose tumors demonstrated at least 50% expression of PD-L1 and did not harbor an EGFR mutation or ALK rearrangement.6 The second approval was in May 2017, based on the randomized phase II KEYNOTE-021 (cohort G) study of 123 patients, for upfront use of pembrolizumab in combination with carboplatin and pemetrexed for patients with metastatic nonsquamous NSCLC, regardless of PD-L1 expression.7 With several other checkpoint inhibitors in development, and trials ongoing of immunotherapy combinations as well as other chemotherapy plus immunotherapy combinations, it would be appropriate to say that a new revolution in frontline lung cancer treatment is underway. However, this revolution also raises questions about what treatment strategy is best for each patient, emphasizing the great importance of a personalized approach.
The international KEYNOTE-024 trial of pembrolizumab was the first phase III trial to show that immunotherapy could replace chemotherapy in the frontline setting for a subset of patients.8 The patients eligible for this trial had to meet the following criteria:
be treatment-naïve, with metastatic nonsquamous or squamous NSCLC, have a PD-L1 tumor proportion score of at least 50% as determined by Dako immunohistochemistry (IHC) 22C3 pharmDx assay, and have no evidence of an EGFR mutation or ALK rearrangement. A total of 305 patients with these characteristics were randomized 1:1 to receive either pembrolizumab 200 mg intravenously (IV) every 3 weeks or investigator’s choice of platinum-based chemotherapy for 4 to 6 cycles. Pemetrexed maintenance was allowed for those patients receiving a pemetrexed-containing regimen. Crossover was also allowed for patients who progressed on chemotherapy. The primary endpoint was progression-free survival (PFS) using RECIST v1.1 criteria, with secondary endpoints of overall survival (OS), objective response rate (ORR), and safety. Patient characteristics between the 2 arms of this study were well balanced. Of the 1653 patient samples eligible for PD-L1 testing, 30% screened positive for at least 50% PD-L1 expression. Although there was a relatively high prevalence of PD-L1 positivity, this did not necessarily mean that 30% of patients were eligible for pembrolizumab frontline therapy. For example, the trial excluded patients with untreated brain metastases, active autoimmune conditions, or active hepatitis B and C, and those with a requirement for steroids or immunosuppressive medications.
The primary endpoint of the study was met, with significantly prolonged PFS in the pembrolizumab arm compared with the chemotherapy arm of 10.3 versus 6 months (HR: 0.50; 95% CI, 0.37-0.68; P <.001).8 The ORR with pembrolizumab was 45% versus 28% with chemotherapy, and the median duration of response was not reached at the time of analysis with pembrolizumab versus 6.3 months with chemotherapy. Despite 44% crossover of the chemotherapy arm to the immunotherapy arm, at the second interim analysis, OS was significantly improved with pembrolizumab versus chemotherapy (HR, 0.60; 95% CI, 0.41-0.89; P = .005), with 70% versus 54% of patients alive at 12 months. This ultimately resulted in the early cessation of the trial by the data safety monitoring committee.
The quality-of-life results were later reported, using the validated European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) Core 30 for global health status and EORTC QLQ-LC13 for lung-cancer–related symptoms, and again, pembrolizumab was significantly favored.9 Fewer treatment-related adverse events (AEs) of any grade were observed in the pembrolizumab arm versus the chemotherapy arm (73.4% vs 90%), with expected autoimmune AEs similar to those previously reported with pembrolizumab. The benefit of pembrolizumab was seen across most subgroups, even when com- pared with those patients who received a pemetrexed-containing regimen. The hazard ratio (HR) point estimate was attenuated for subgroups of female patients and nonsmokers, though the latter group included smaller numbers of patients. The KEYNOTE-024 study provided the results to propel a novel treatment to replace chemotherapy in the first-line setting for a relevant subset of patients who were EGFR- and ALK-negative and had positive expression of the PD-L1 biomarker.
At the same time the KEYNOTE-024 trial was being conducted, the CheckMate-026 trial was underway, assessing nivolumab in the frontline setting in treatment-naïve patients with advanced NSCLC, no EGFR mutation or ALK rearrangement, and at least 1% PD-L1 expression as assessed by the Dako IHC 28-8 pharmDx assay.10 The IHC antibody used and PD-L1 expression threshold used for testing differed from that of the KEYNOTE-024 pembroli- zumab trial, which required at least a 50% cutoff. A total of 541 patients were randomized 1:1 to receive nivolumab 3 mg/kg IV every 2 weeks, or histology-dependent standard first-line platinum doublet chemotherapy. Crossover was allowed. The primary endpoint was PFS as determined by RECIST v1.1 criteria, though the criteria were examined using a 5% PD-L1 threshold instead of the 1% threshold required for eligibility. Secondary endpoints included PFS in PD-L1 expression greater than or equal to 1%, OS, and ORR. Baseline characteristics showed a higher female predominance in the chemotherapy arm; otherwise, the treatment arms were well balanced.
The results of the trial were negative, with no difference in PFS at the 5% PD-L1 expression threshold. The median PFS was 4.2 months in the nivolumab arm versus 5.9 months in the chemo- therapy arm (HR, 1.15; 95% CI, 0.91-1.45; P = .251).10 There was also no difference in OS, with a median OS of 14.4 months in the nivolumab arm versus 13.2 months in the chemotherapy arm (HR, 1.02; 95% CI, 0.80-1.30). In patients with PD-L1 expression of at least 5%, ORRs were 26% and 34% in the nivolumab and chemotherapy arms, respectively. Also, more patients had a best response of progressive disease in the nivolumab arm versus the chemotherapy arm (28% vs 10%). Of patients who attained a response, however, the median duration of response was 12.1 months in the nivolumab arm versus 5.7 months in the chemo- therapy arm, suggesting a prolonged benefit of immunotherapy in the patients who do respond. Interestingly, 60% of patients in the chemotherapy arm had subsequent nivolumab therapy versus only 44% in the nivolumab arm eventually receiving systemic therapy, suggesting that a majority of patients in the nivolumab arm did not have the opportunity to later receive a potentially effective treatment. The negative results for PFS and OS were seen across almost all subgroups, even in those patients with high PD-L1 expression of at least 50%, with unstratified HRs of 1.07 and 0.90 for PFS and OS, respectively.
KEYNOTE-024 and CheckMate-026
So how can the differences between the results of the KEYNOTE-024 pembrolizumab and CheckMate-026 nivolumab trials be explained? Although there is no clear explanation, several observations are important to consider. First, comparing the baseline characteristics of the trials, there was a higher percentage of nonsmokers in the immunotherapy arm of the CheckMate-026 trial than in the immunotherapy arm of the KEYNOTE-024 trial (11.1% vs 3.2%), although the percentage of nonsmokers was well balanced between the nivolumab and chemotherapy arms.10 In other nonsmoker subgroup analyses, including in these studies, there is more limited benefit demonstrated for immunotherapy over chemotherapy. This may correlate to the hypothesis that a higher mutational burden is related to clinical benefit from immune checkpoint inhibitors in lung cancer.11 Regarding radiation, in the CheckMate-026 trial, 38% to 40% of patients in both arms, surprisingly, had received prior radiation therapy, despite being systemic treatment-naïve in the advanced setting.10 Prior radiation was not reported in the KEYNOTE-024 study, although patients who received thoracic radiation of greater than 30 Gy within 6 months of the trial start were excluded.8 It is unclear how this may have played a role in the differences observed between the trial outcomes, but it is a notable difference.
Second, with regard to PD-L1 expression, in CheckMate-026, the threshold for positivity was lower than that of the KENOTE-024 trial. Despite the 5% threshold for PD-L1 expression being a stratification factor at randomization in the CheckMate-026 study and being balanced between both arms, there were a greater proportion of patients with high PD-L1 expression in the chemo- therapy arm starting at the 25% threshold.10 In the CheckMate-057 trial assessing nivolumab in the second-line setting in patients with nonsquamous advanced NSCLC, PD-L1 positivity was not required to enroll, and there was a significant correlation between a higher PD-L1 expression level and more pronounced benefit to immunotherapy starting at the 1% threshold.3
A thought-provoking exploratory subset analysis of 58% of patients in the CheckMate-026 study showed that high tumor mutation burden (TMB) might be a more effective biomarker.12 In patients with high TMB (≥ 243 somatic mutations), nivolumab showed a trend for improved PFS (HR, 0.62; 95% CI, 0.38-1.00) and ORR compared with chemotherapy. The contrary was true for patients with low or medium TMB, in which nivolumab was inferior to chemotherapy for PFS (HR, 1.82; 95% CI, 1.30-2.55). Surprisingly, there was no association between TMB and PD-L1 expression for patients in this study who all had tumors with PD-L1 expression ≥1%, suggesting TMB may be a better biomarker. In addition, patients with both high TMB and high PD-L1 expression ≥50% derived the most benefit with nivolumab. OS, however, was similar regardless of TMB, although significant crossover may account for this. Additional biomarkers besides PD-L1 expression may be useful in the future as predictors of response to immunotherapy, and patient selection may remain critical in terms of which biomarkers are most applicable.
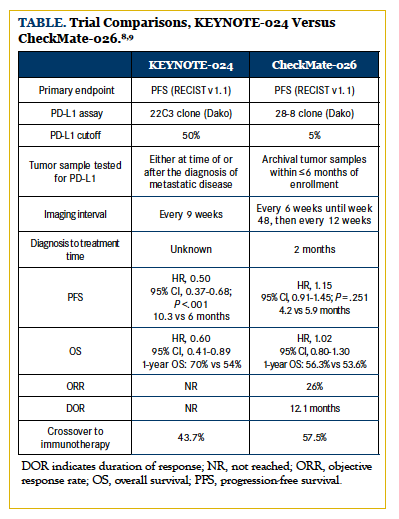
Furthermore, although both trials allowed crossover, in CheckMate-026, 58% of patients crossed over to the nivolumab arm versus 44% to the pembrolizumab arm in the KEYNOTE-024 trial, potentially attenuating survival data for the CheckMate-026 study. Other trial design factors that may have played a role include the time point at which PD-L1 was tested, imaging frequency for PFS endpoint, and the time from diagnosis to first treatment. The Table summarizes these trial comparisons and differences. Despite the results, nivolumab continues to remain a reliable option in the second-line setting and beyond, with other PD-1 and PD-L1 inhibitors currently being testing as frontline agents.13
With KEYNOTE-024 using a 50% PD-L1 cutoff and Check- Mate-026 using a 5% cutoff for the primary endpoint analysis, an important question to be answered focuses on the patients who fall between these levels. Would an advanced NSCLC patient with PD-L1 expression of 40% benefit from frontline immunotherapy alone? This may remain an important consideration for future studies. Also, with a different PD-L1 assay used for each approved checkpoint inhibitor, how can accuracy and reproducibility among the assays be guaranteed? In the International Association for the Study of Lung Cancer’s Blueprint PD-L1 IHC Assay Comparison Project, 39 NSCLC tumors were stained with 4 available PD-L1 IHC assays used previously in clinical trials (22C3 with pembrolizumab, 28-8 with nivolumab, SP142 with atezolizumab, and SP263 with durvalumab).14 Analytical concordance was demonstrated among the 22C3, 28-8, and SP263 assays; however, the SP142 assay, used in trials with atezolizumab, stained fewer tumor cells, suggesting an underestimation of PD-L1 expression. In addition, for 37% of cases, depending on the assay used, a different PD-L1 classification was made. Though pembrolizumab is currently the only drug for which PD-L1 testing is a companion diagnostic, this will likely change in the future. Reproducibility among available assays is vital to avoid PD-L1 expression misclassification and, in turn, ensure the appropriate use of PD-1/PD-L1 checkpoint inhibitors. Other predictive biomarkers such as TMB may also become more relevant.
With the success of first-line pembrolizumab monotherapy in the KEYNOTE-024 trial, interest has risen significantly in combining immunotherapy with chemotherapy or with other immune therapies, such as CTLA-4 antibodies. The KEYNOTE-021 (cohort G) trial suggests that platinum-based chemotherapy in the frontline setting may not be so passé for a subset of patients with nonsquamous NSCLC. This was a phase II study of 123 patients with untreated stage IIIB/IV nonsquamous NSCLC without an EGFR mutation or ALK rearrangement and no PD-L1 requirement, randomized 1:1 to pembrolizumab plus 4 cycles of carboplatin plus pemetrexed versus 4 cycles of carboplatin plus pemetrexed alone, with pemetrexed permitted as maintenance therapy in both arms.7 The primary endpoint was ORR, with secondary endpoints being PFS, OS, safety, and the relationship between response and PD-L1 expression. The results showed a significant (26%) improvement in ORR for the pembrolizumab plus chemotherapy combination compared with chemotherapy alone, at 55% versus 29% (95% CI, 9%-42%; P = .0016). The response rates were similar if patients were above or below the 1% PD-L1 threshold in the combination arm.
However, patients with 50% or greater PD-L1 expression in the combination arm had an ORR of 80%. On the other hand, this segment represented only 20 patients, and thus was too small from which to draw a definitive conclusion.
As a secondary endpoint, PFS was also significantly improved at 13 months in the combination arm versus 8.9 months in the chemotherapy-alone arm (HR, 0.53; 95% CI, 0.31-0.91). At a median follow-up of 10.6 months, OS was not improved, though a 52% crossover rate was noted. The incidence of grade 3 or worse treatment-related AEs was 39% in the combination group versus 26% in the chemotherapy group.7 Under the FDA’s accelerated approval regulation, the combination of pembroli- zumab plus carboplatin and pemetrexed achieved first-line approval in May 2017, in patients with metastatic nonsquamous NSCLC regardless of PD-L1 expression. The confirmatory phase III KEYNOTE-189 trial, assessing the use of carboplatin or cisplatin and pemetrexed plus or minus pembrolizumab for nonsquamous histology, is underway, with continued approval of combination pembrolizumab contingent upon a demonstrated positive clinical benefit.15 With regard to squamous histology, the phase III KEYNOTE-407 trial will assess carboplatin and paclitaxel or nab-paclitaxel plus or minus pembrolizumab in patients with advanced squamous NSCLC.16
It remains unclear at this time whether there will be great advantage to combining platinum-based chemotherapy and immuno- therapy as first-line treatment rather than sequencing it, especially if there proves to be no OS benefit. It is also not known yet whether these results will translate in the phase III setting. Since the approval was granted for use regardless of the presence of PD-L1 expression, it is unclear if there will be an advantage to combination chemotherapy and pembrolizumab for patients who have tumor PD-L1 expression of at least 50% and who achieve reasonable response rates and PFS times with pembrolizumab alone. Nonetheless, the combination is a viable option for patients with nonsquamous histology. Important considerations for use include patient performance status and ability to tolerate a potentially higher chance of AEs, tumor burden, scenarios in which a patient with nonsquamous NSCLC has negative (<1%), or intermediate (1% to 49%) tumor PD-L1 expression and needs a rapid, higher chance of response in the frontline setting, and patient preference following an informed discussion.
Conclusion
The current landscape of frontline therapy for advanced NSCLC is evolving rapidly and now includes immunotherapy. The major breakthrough was pembrolizumab, now with FDA regulatory approval in the first-line setting as a monotherapy and with FDA accelerated approval in combination with carboplatin-pemetrexed chemotherapy. In the KEYNOTE-024 trial, pembrolizumab improved PFS and OS in the upfront setting in patients with at least 50% PD-L1 expression and no EGFR mutation or ALK rearrangement. This has had major treatment implications, now allowing a significant percentage of patients with metastatic NSCLC to proceed directly to immunotherapy alone. For patients with nonsquamous histology irrespective of PD-L1 expression, based on the phase II KEYNOTE-021 (cohort G) trial, pembrolizumab plus carboplatin and pemetrexed is now an option, showing improvement in ORR and PFS, but not in OS, compared with chemotherapy alone. Other early-phase combination immunotherapy trials with the CTLA-4 inhibitor ipilimumab have been promising, paving the way for several larger phase III combination trials that are actively recruiting or ongoing.17,18 The disappointing results of nivolumab in the frontline setting can perhaps be explained by a host of factors, though there is not a single clear explanation. Patient selection may have been critical, and there may be better biomarkers to be identified, such as TMB. Improved standardization of PD-L1 assays will require future attention. All in all, though the use of platinum-only chemotherapy in metastatic disease is not passé, as almost all patients will receive and derive benefit from chemotherapy at some juncture in their disease course, this paradigm is certainly changing in the frontline setting at least.
Author affiliations: Sukhmani K. Padda, MD, and Jeffrey Zweig, MD, are with Stanford University and Stanford Cancer Institute in Stanford, California.
Address correspondence to: Sukhmani K. Padda, MD, Stanford University and Stanford Cancer Institute, Department of Medicine, Division of Oncology, 875 Blake Wilbur Dr, Stanford, CA 94305-5826. E-mail: [email protected].
Financial disclosures: Sukhmani K. Padda, MD, has participated in paid advisory boards and consultancies with Janssen Pharmaceuticals, G1 Therapeutics, and AstraZeneca and has received honoraria from Physicians’ Education Resources®, LLC, and the International Association for the Study of Lung Cancer.
References
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542-2550.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa- 1504627.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi: 10.1016/S0140-6736(15)01281-7.
- Rittmeyer A, Barlesi F, Waterkamp D, et al; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X.
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 6.2017). nccn.org/professionals/physician_gls/pdf/ nscl.pdf. Accessed May 30, 2017.
- Langer CJ, Gadgeel SM, Borghaei H, et al; KEYNOTE-021 investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497-1508. doi: 10.1016/S1470- 2045(16)30498-3.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
- Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. PL04a.01 - Health-related quality of life for pembrolizumab vs chemotherapy in advanced NSCLC with PD-L1 TPS ≥50%: data from KEYNOTE-024. J Thorac Oncol. 2017;12(1):S8-S9. doi: 10.1016/j.jtho.2016.11.010.
- Socinski M, Creelan B, Horn L, et al. CheckMate 026: a phase 3 trial of nivolumab vs investigator’s choice (IC) of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for stage iv/ recurrent programmed death ligand 1 (PD-L1)−positive NSCLC. Ann Oncol. 2016;27(suppl_6):LBA7_PR. https://doi.org/10.1093/ annonc/mdw435.39.
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non- small cell lung cancer. Science. 2015;348(6230):124-128. doi: 10.1126/ science.aaa1348.
- Peters S, Creelan B, Hellmann MD, et al. Impact of tumor mutation burden on the efficacy of first-line nivolumab in stage IV or recurrent non-small cell lung cancer: an exploratory analysis of Check- Mate 026. AACR 2017. Abstract #CT082.
- A phase III, open label, randomized study of atezolizumab (an- ti-PD-L1 antibody) compared with a platinum agent (cisplatin or carboplatin) in combination with either pemetrexed or gemcitabine for PD-L1-selected, chemotherapy-naive patients with stage IV non-squamous or squamous non–small-cell lung cancer. clinicaltrials.gov/ct2/ show/NCT02409342?term=NCT02409342&rank=1. Updated April 2015. Accessed June 2017.
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12(2):208-222. doi: 10.1016/j.jtho.2016.11.2228.
- A randomized, double-blind, phase III study of platinum + pemetrexed chemotherapy with or without pembrolizumab (MK-3475) in first-line metastatic non-squamous non–small-cell lung cancer subjects (KEYNOTE-189). clinicaltrials.gov/ct2/show/NCT02578680?term=NCT02578680&rank=1. Updated October 2015. Accessed June 2017.
- A randomized, double-blind, phase III study of carboplatin-paclitaxel/nab-paclitaxel chemotherapy with or without pembrolizumab (MK-3475) in first-line metastatic squamous non–small-cell lung cancer subjects (KEYNOTE-407). clinicaltrials.gov/ct2/show/ NCT02775435?term=NCT02775435.&rank=1. Updated May 2016. Accessed June 2017.
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multi- cohort study. Lancet Oncol. 2017;18(1):31-41. doi: 10.1016/S1470- 2045(16)30624-6.
- An open-label, randomized phase 3 trial of nivolumab, or nivolumab plus ipilimumab, or nivolumab plus platinum doublet chemotherapy versus platinum doublet chemotherapy in subjects with chemotherapy-naïve stage IV or recurrent non– small-cell lung cancer (NSCLC). clinicaltrials.gov/ct2/show/ NCT02477826?term=NCT02477826&rank=1. Updated June 2015. Accessed June 2017.





