Introduction
The treatment of advanced epidermal growth factor receptor (EGFR) mutant non-small–cell lung cancer (NSCLC) has been revolutionized by continuous developments in the field of targeted therapy. First- or second-generation EGFR kinase inhibitors have become standard initial therapy for this disease.1-4 However, the considerable clinical benefit of these agents is limited by the inevitable development of acquired resistance.5 The evaluation and management of acquired resistance to initial therapy with EGFR kinase inhibitors has thus emerged as a key clinical challenge in the treatment of advanced EGFR mutant NSCLC.Advances in the understanding of the mechanisms that underpin acquired resistance to EGFR kinase inhibitors have recently begun to yield novel targeted therapies. This is best exemplified by the recent development of third-generation EGFR kinase inhibitors capable of treating EGFR T790M-mediated acquired resistance.6,7 The recent FDA approval of one agent in this class, osimertinib, has increased treatment options available to patients, but has magnified the complexity of managing acquired resistance to EGFR kinase inhibitors. Here, we review strategies for the optimal management of acquired resistance to initial EGFR kinase inhibitors in EGFR mutant NSCLC.
Mechanisms of Acquired Resistance to EGFR Kinase Inhibitors
Acquired resistance to EGFR kinase inhibitors inevitably occurs in advanced EGFR mutant NSCLC. This resistance may occur via the outgrowth of a pre-existing resistant subclone in some cases, or acquisition of a new resistance mutation in a previously sensitive cancer cell.8 Recent data have suggested that both processes may occur in patients.9 Regardless of its cellular origin, genetic studies of rebiopsy specimens derived from EGFR mutant NSCLC patients with acquired resistance have revealed multiple mechanisms underpinning resistance.10-12 The most common mechanism of acquired resistance is the EGFR T790M gatekeeper mutation, which is detectable in approximately 50-60% of patients, and impairs the binding of first- and second-generation EGFR kinase inhibitors to the adenosine triphosphate (ATP)-binding pocket of mutant EGFR.10-12 Importantly, only 65-70% of these patients will respond to the third-generation EGFR kinase inhibitor, osimertinib, implying that T790M may represent a minor subpopulation in these patients and/or that additional resistance mechanisms may be present.13,14 An alternative genetic mechanism of resistance is acquired-MET amplification, which occurs in 5-10% of patients.10-12 Rarer mechanisms such as small-cell lung cancer transformation have also been reported.10,11 Pharmacokinetic resistance may also occur in the instance of isolated brain metastases, where sufficient concentrations of an otherwise effective EGFR kinase inhibitor cannot penetrate the blood brain barrier. The rational selection of subsequent therapy hinges upon careful selecton of the time point at which to discontinue initial EGFR kinase inhibitor therapy, as well as careful determination of the mechanism underpinning acquired resistance in a given patient.Defining Clinically Significant Progression
The first evidence of the emergence of acquired resistance to an EGFR kinase inhibitor, is commonly, asymptomatic radiographic progression. Objective clinical and radiographic definitions of acquired resistance have been previously proposed for the purpose of defining this clinical entity to facilitate clinical trials. The majority of these definitions utilize objective radiographic progression per, Response Evaluation Criteria In Solid Tumors (RECIST), as a key indicator of acquired resistance. This is an important tool to identify patients for clinical trials as well as an important study end point. However, the initial development of acquired resistance may manifest as subtle and indolent radiographic progression that does not necessitate an immediate change in therapy.5 The clinical decision to change treatment in the context of acquired resistance to EGFR kinase inhibitors is nuanced and must take into consideration many important factors related to both the patient and the biology of their underlying disease.The rate and pattern of progression observed in EGFR mutant advanced NSCLC with acquired resistance is variable and potentially related to the underlying mechanism of acquired resistance. EGFR T790M-positive disease has, in particular, been reported to exhibit a more indolent course than other subtypes.15 Patients with more indolent disease may benefit from remaining on EGFR kinase inhibitor therapy after the emergence of acquired resistance with the rationale that acquired resistance is a heterogeneous process, and that the need for initiating second-line therapy may be delayed by continuing initial therapy.16 The distinction between the identification of acquired resistance and clinically significant progression necessitating treatment change is, therefore, key (Table 1).
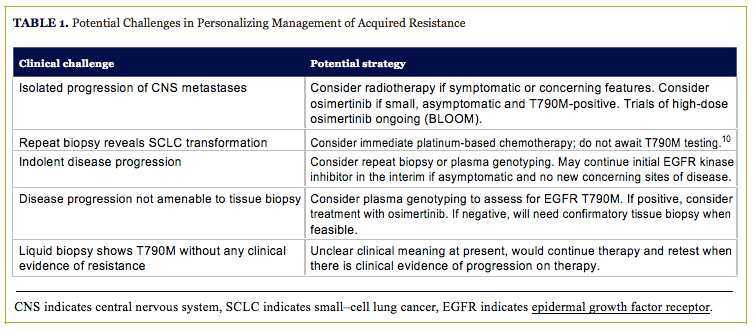
The ASPIRATION study recently reported on the feasibility of continuing EGFR kinase inhibitor therapy beyond initial radiographic progression. This single-arm phase II study of EGFR mutant advanced NSCLC treated with first-line erlotinib and allowed patients to continue erlotinib, after initial progression, at the discretion of the treating physician. The patients in this study who continued on erlotinib post-progression were more likely to have good performance status, longer initial duration of response, greater depth of response, and possess isolated brain metastases compared to those who discontinued treatment at initial progression. Those who continued on erlotinib were able to remain on therapy for a median of an additional 3.1 months before discontinuing therapy due to symptomatic disease progression necessitating a change of therapy.16 However, caution must be exercised in interpreting the results of this study, particularly given the lack of a comparator arm and the high potential for selection bias, as well as confounding by indication and disease severity inherent in the study design.17 Previous retrospective studies have also found that post-progression therapy, with an EGFR kinase inhibitor can prolong survival compared to immediately switching to second-line therapy, and that the use of locally ablative therapy with EGFR kinase inhibitor therapy can delay the need for second-line therapy in the context of acquired resistance.18-20 However, no added benefit has been observed in combining EGFR inhibition with subsequent second-line combination chemotherapy.21 These studies underscore the feasibility of postprogression therapy in patients who have good performance status and asymptomatic indolent progression of minimal residual disease.
Resistance Biopsy and the Emerging Role of Plasma Genotyping
The selection of systemic therapy for acquired resistance now hinges on identifying the molecular mechanism that is driving disease progression.5 This may be assessed by a repeat biopsy and molecular testing for EGFR T790M, as well as MET amplification and histological analysis for evidence of small-cell transformation. Repeat biopsies are inherently limited by their invasive nature, risk of complications, and potential for delaying subsequent therapy. Acquired resistance to EGFR kinase inhibitors is also increasingly recognized as a heterogeneous process across meta-static sites in a given patient.22 Therefore, the resistance biopsy of a single metastatic site may not be representative of the resistance mechanism at work in other metastatic sites and, in particularly unlucky situations, could hypothetically miss the resistance mechanism present at the majority of sites.Plasma genotyping of cell-free DNA (cfDNA) allows for the rapid and noninvasive detection of EGFR T790M while avoiding many of the inherent limitations of repeat tissue biopsy.23 Various platforms for plasma genotyping exist with variable levels of validation. A prospective validation of plasma genotyping utilizing a droplet digital polymerase chain reaction (ddPCR)-based platform has recently been completed demonstrating that this assay exhibits high specificity for the detection of EGFR and KRAS driver mutations. Interestingly, the assay detected some EGFR T790M mutations missed by tissue genotyping that is likely secondary to heterogeneity of resistance mechanism across metastatic sites.24 Plasma genotyping may be particularly useful in patients where repeat tissue biopsy is not feasible at resistance, while also having the potential to detect EGFR T790M mutations that are missed by standard tissue genotyping. Although a positive result from these assays is actionable, caution should be exercised in interpreting negative plasma results. A negative result may imply the absence of a mutation or merely that a patient’s tumor is not shedding cfDNA at detectable levels, thus necessitating a confirmatory tissue biopsy to rule out a false negative plasma result.
Changing Therapy
Patients with clinically significant disease progression and evidence of an EGFR T790M mutation are good candidates for treatment with the third-generation EGFR kinase inhibitor, osimertinib. A recent single-arm study of 253 patients with acquired resistance to EGFR kinase inhibitors treated with osimertinib demonstrated an impressive objective response rate (ORR) of 61% and progression-free survival (PFS) of 9.6 months among EGFR T790M-positive patients.6 The results of this study have led to the accelerated regulatory approval of osimertinib, while a randomized phase III study is ongoing. The ease of initiating second-line therapy with a highly active oral kinase inhibitor, such as osimertinib, renders it a more appealing option than moderately active systemic chemotherapy. Therefore, practitioners may opt to utilize it earlier in patients with T790M-mediated acquired resistance and perhaps even in the instance of indolent asymptomatic progression.Treatment in patients with non-T790M mediated acquired resistance requires careful consideration of individual patient and disease characteristics. Patients with localized disease progression or isolated brain metastases could be considered for local radiotherapy and possible continuation of initial EGFR kinase inhibitor therapy.19 Isolated progression of central nervous system (CNS) metastases may also be amenable to treatment with osimertinib, a strategy currently being investigated in the BLOOM trial which contains an arm examining high-dose osimertinib in CNS metastases regardless of tumor T790M-status (NCT02228369). Any T790M-negative disease progression should prompt consideration of standard secondline therapy with intravenous platinum-based chemotherapy, especially in the instance of small cell transformation (Table 1).5,10 Patients with MET-amplified tumors may benefit from combination treatment with an EGFR and MET inhibitor.25 This strategy is currently under investigation in multiple clinical trials, although anecdotal evidence exists of clinical responses to standard inhibitors such as the combination of erlotinib and crizotinib. Strong consideration should be given to available clinical trial options in EGFR T790M-negative patients.
Conclusion
Acquired resistance is a predictable and unavoidable waypoint in the initial treatment of EGFR mutant NSCLC. The natural history of resistant disease is variable and presents multiple options for treatment based on clinical parameters and the molecular mechanism underpinning resistance in a given patient. Strong consideration should be given to postprogression EGFR kinase inhibitor therapy in asymptomatic patients with indolent disease. Careful analysis of resistance mechanisms at the time of clinically significant disease progression will allow for the smooth transition to approved therapies in the instance of T790M-mediated resistance or the consideration of clinical trials in MET-amplified tumors. Standard chemotherapy, locally ablative therapies, and clinical trials are options for the patients with T790M-negative tumors.Affiliations: Adrian G. Sacher, MD, is from Columbia University/New York-Presbyterian Hospital, New York, NY. Geoffrey R. Oxnard, MD, is from Lowe Center for Thoracic Oncology, Dana-Farber Cancer Institute, Boston, MA, and Brigham and Women’s Hospital & Harvard Medical School, Boston, MA.
Disclosures: Dr Sacher has received travel funding from AstraZeneca and Genentech-Roche. Dr Oxnard has received consulting fees from Ariad, AstraZeneca, Boehringer-Ingelheim, Genentech, and Inivata, and honoraria from AstraZeneca, Boehringer-Ingelheim, and Chugai.
Address correspondence to: Geoffrey R. Oxnard, MD, Dana-Farber Cancer Institute, 450 Brookline Ave., Dana 1234, Boston, MA 02215-5450. Phone: 617-632-6049; Fax: 617-632-5786; E-mail: [email protected]
References
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi: 10.1056/NEJMoa0810699.
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi: 10.1016/S1470-2045(11)70393-X.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small–cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-2388. doi: 10.1056/ NEJMoa0909530.
- Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14(10):953-961. doi: 10.1016/S1470-2045(13)70355-3.
- Sacher AG, Janne PA, Oxnard GR. Management of acquired resistance to epidermal growth factor receptor kinase inhibitors in patients with advanced non-small cell lung cancer. Cancer. 2014;120(15):2289-2298. doi: 10.1002/cncr.28723.
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689-1699. doi: 10.1056/NEJMoa1411817.
- Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;372(18):1700-1709. doi: 10.1056/NEJMc1506831.
- Oxnard GR. The cellular origins of drug resistance in cancer. Nat Med. 2016;22(3):232-234. doi: 10.1038/nm.4058.
- Hata AN, Niederst MJ, Archibald HL, et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med. 2016;22(3):262-269. doi: 10.1038/nm.4040.
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003.
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240-2247. doi: 10.1158/1078-0432.CCR-12-2246.
- Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17(5):1169-1180. doi: 10.1158/1078-0432.CCR-10-2277.
- Oxnard G, Thress K, Alden R, et al. Plasma genotyping for predicting benefit from osimertinib in patients with advanced NSCLC. European Lung Cancer Conference (ELCC); 2016; Geneva, Switzerland.
- Yang JC-H, Ramalingam S, Janne P, et al. Osimertinib (AZD9291) in pre-treated patients with T790M-positive advanced NSCLC: updated Phase I and pooled Phase II results. European Lung Cancer Conference (ELCC); 2016; Geneva, Switzerland.
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17(6):1616-1622. doi: 10.1158/1078-0432.CCR-10-2692.
- Park K, Yu CJ, Kim SW, et al. First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in Asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: the ASPIRATION study. JAMA Oncol. 2016;2(3):305-312. doi: 10.1001/jamaoncol.2015.4921.
- Gandara DR, Redman M, Hirsch FR. Postprogression prolongation of survival in EGFR-mutated lung cancer: reconciling the ASPIRATION and IMPRESS trials. JAMA Oncol. 2016;2(3):300-301. doi: 10.1001/jamaoncol.2015.4920.
- Nishie K, Kawaguchi T, Tamiya A, et al. Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: a retrospective analysis for Japanese patients with activating EGFR mutations. J Thorac Oncol. 2012;7(11):1722-1727. doi: 10.1097/JTO.0b013e31826913f7.
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7(12):1807-1814. doi: 10.1097/ JTO.0b013e3182745948.
- Lo PC, Dahlberg SE, Nishino M, et al. Delay of treatment change after objective progression on first-line erlotinib in epidermal growth factor receptor-mutant lung cancer. Cancer. 2015;121(15):2570-2577. doi: 10.1002/cncr.29397.
- Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on firstline gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16(8):990-998. doi: 10.1016/S1470-2045(15)00121-7.
- Sundaresan TK, Sequist LV, Heymach JV, et al. Detection of T790M, the acquired resistance EGFR mutation, by tumor biopsy versus noninvasive blood-based analyses. Clin Cancer Res. 2016;22(5):1103-1110. doi: 10.1158/1078-0432.CCR-15-1031.
- Paweletz CP, Sacher AG, Raymond CK, et al. Bias-corrected targeted next-generation sequencing for rapid, multiplexed detection of actionable alterations in cell-free DNA from advanced lung cancer patients. Clin Cancer Res. 2015. doi: 10.1158/1078-0432.CCR-15-1627-T.
- Sacher A, Dahlberg S, Paweletz C, et al. Prospective validation of rapid plasma genotyping as a sensitive and specific tool for guiding lung cancer care. JAMA Oncol. 2016. doi:10.1001/ jamaoncol.2016.0173
- Dietrich MF, Yan SX, Schiller JH. Response to crizotinib/ erlotinib combination in a patient with a primary EGFR-mutant adenocarcinoma and a primary c-met-amplified adenocarcinoma of the lung. J Thorac Oncol. 2015;10(5):e23-25. doi: 10.1097/ JTO.0000000000000448.





