Introduction
Given the advances in metastatic castration-resistant prostate cancer (mCRPC) in the last 5 years, it is appropriate to review the data and take stock of where we have been and where we need to go. There is no doubt that tremendous progress has been made, and that new trials have changed the way we approach this disease. While we laud this progress, it is sometimes easy to forget the shortcomings and how much further we need to go. No one with mCRPC will be cured, and our efforts as a whole have been modest. No comparative trials have been published, and few are in progress. No combination is known to be superior to a single agent, and no sequence is known to be superior to any other sequence. This article will review the data leading to regulatory approvals, but also highlight some of the questions and shortcomings associated with each agent. Clearly there is a need for further and active investigation in this space.
Chemotherapy in Newly Diagnosed mCRPC
Ten years after the publication of 2 seminal articles detailing the survival advantage of docetaxel over mitoxantrone for patients with mCRPC1,2 and its US Food and Drug Administration (FDA) approval for use in mCRPC, docetaxel has found another potential place in the treatment of prostate cancer. The ECOG CHAARTED trial3 randomized in a 1:1 fashion 790 men with hormone-sensitive mCRPC to usual treatment consisting of androgen-deprivation therapy (ADT) or to ADT plus 6 cycles of docetaxel chemotherapy given at 75 mg/m2 every 3 weeks. The primary endpoint was overall survival (OS). The median age was 63 years, and the majority of the patients had an ECOG performance status (PS) of 0 or 1. Median OS in the ADT group was 42.3 months and in the ADT-plus-docetaxel group it was 52.7 months, giving a 10.4-month survival advantage to the use of chemotherapy. Patients were stratified based on having high-volume disease (visceral disease and/or ≥4 bone lesions, with at least 1 outside of the pelvis and vertebral column) or low-volume disease. Patients in the high-volume group had a median OS of 32.2 months compared with 49.2 months in the ADT-plus-docetaxel group (hazard ratio [HR], 0.6; P = .0006). In the low-volume group, the median OS was not met, so further follow-up will be required.3
It is important to note that the GETUG-15 trial4 was conducted in a similar patient population, but without demonstrating a benefit to added docetaxel. Differences in the 2 trials are readily apparent. The GETUG-15 trial was much smaller (N = 385 vs 790), utilized up to 9 cycles of docetaxel (vs ≤6), and had 176 death events versus 237 for CHAARTED. The GETUG-15 control group had a median survival of 54 months compared with 44 months for CHAARTED, and the overall GETUG-15 study had a median prostate-specific antigen (PSA) at entry of 26 ng/mL versus 53 ng/mL in CHAARTED. Taken together, the smaller GETUG-15 trial coupled with the better prognosis of enrolled patients may account for the differences in the trial results. More follow-up is needed for both trials.
STAMPEDE5 is a third trial that has recently reported results. In this trial, 2962 men with high-risk, locally advanced or mCRPC who had started ADT were randomized to standard of care (SOC), which was at least 3 years of ADT; SoC plus docetaxel given at a dosage of 75 mg/m2 every 3 weeks for 6 cycles; SOC plus zoledronic acid; or SOC plus zoledronic acid and docetaxel. The proportion of patients with metastatic disease was 61%. In the overall trial, the median OS was increased by 10 months (67 months vs 77 months), favoring the use of docetaxel with an HR for OS of 0.76. For those with metastatic disease, the HR was 0.73, and for those with nonmetastatic disease, the HR was 1.01 (with many fewer events in the nonmetastatic arm). In the metastatic subset, the docetaxel-plus-ADT subset median survival was 65 months compared with 43 months for those treated with ADT alone. In other words, those with metastatic disease receiving docetaxel in the hormone-sensitive setting had a 22-month longer median survival.
The STAMPEDE results confirm the results of the CHAARTED trial and will change the standard of care for newly diagnosed mCRPC. Regardless, not all patients are appropriate for chemotherapy, and not all patients will consent to chemotherapy. However, in patients who present with metastatic disease, particularly those with visceral disease and 4 or more bone metastases, chemotherapy should be offered. Although the standards should change, what should become of patients who are chemo-intolerant or otherwise not candidates for chemotherapy? There is a strong rationale for combining ADT with nonchemotherapeutic agents. ADT combined with radium-223, enzalutamide, ARN-509, ODM-201, or abiraterone may also be effective, and even better tolerated. New trials to address these issues are either ongoing or proposed. Regardless, the possibility that ADT monotherapy will remain the SoC is remote. Times are changing.
Docetaxel
Docetaxel was approved for use in men with mCRPC in 2004 as a result of 2 studies published in the same issue of The New England Journal of Medicine that year (Table 1). The TAX 3271 study randomized 1006 men with mCRPC to receive either docetaxel or mitoxantrone. The primary endpoint of OS was met with a 2.4-month improvement in OS favoring docetaxel. All patients were given prednisone 5 mg twice a day and were premedicated with dexamethasone in the following way: 8 mg orally given 12 hours, 3 hours, and 1 hour prior to chemotherapy for the every-3-week regimen, and 8 mg orally given 1 hour prior to chemotherapy for the weekly regimen. PSA response, defined as a 50% decline in PSA, was also superior in the docetaxel groups, with 45% in the 3-weekly regimen, 48% in the weekly regimen, and 32% in the mitoxantrone group.
SWOG 99162 randomized 770 men with mCRPC to a combination of docetaxel and estramustine or to mitoxantrone. The primary endpoint was met with an improvement in OS of 1.9 months favoring docetaxel.
Docetaxel 75 mg/m2 every 3 weeks is the FDA-approved dosing, and given that estramustine was associated with toxicity without clear additional efficacy, estramustine is rarely used today. An alternate docetaxel dosing regimen of 50 mg/m2 given every 14 days was evaluated in a randomized trial involving 361 patients.6 In this trial, 184 patients were randomized to docetaxel 75 mg/m2 every 3 weeks, and 170 patients were randomized to docetaxel 50 mg/m2 every 2 weeks. Both regimens were given in combination with oral prednisolone 10 mg daily. The primary endpoint was time to treatment failure (TTF). The TTF in the every-2-week regimen was 5.6 months compared with 4.9 months for the every-3-week regimen, with an HR of 1.3 for the 3-week arm (P = .014). OS was a secondary endpoint. Interestingly, the HR for OS was 1.4 (P = .021) for the 3-week group, indicating a worse survival for the standard FDA-approved schedule. There were also more adverse events (AEs) in the 3-weekly regimen including neutropenia (53% vs 36%) and neutropenic fever (14% vs 4%), and infection with neutropenia (24% vs 6%). This is another reasonable dosing schedule for patients designated to receive docetaxel, and we note that this is the only trial with a head-to-head comparison of 2-weekly versus 3-weekly docetaxel that demonstrates a survival advantage. Although the limitations of OS being a secondary endpoint are apparent, this trial demonstrates a provocative result, and the AE profile in the 2-weekly arm at 50 mg/m2 demonstrates clear tolerability. Docetaxel plays a key role in prostate cancer, but many patients die without having seen docetaxel, and thus many men will never benefit from this therapy.
Sipuleucel-T
Sipuleucel-T is an immune-modulating agent best described as an autologous cellular immunotherapy generated after apheresis of the patient’s own immune cells. The patient’s peripheral blood mononuclear cells are treated with a prostatic acid phosphatase–granulocyte macrophage colony-stimulating factor (PAP-GM-CSF) fusion protein in addition to various other cytokines to generate the final product. The administered dose for the patient consists of a minimum of 50 × 106 CD54+ cells given intravenously. This treatment was FDA-approved in 2010 for use in patients with mCRPC, based in part on the results of the pivotal IMPACT study7 that randomized 512 men to either sipuleucel-T or placebo consisting of blood mononuclear cells untreated with PAP-GM-CSF. The sipuleucel-T arm had a 4.1-month OS advantage compared with placebo. There was, however, no progression-free survival (PFS) advantage for the use of sipuleucel-T, with the median time to objective disease progression of 3.7 months in the sipuleucel-T group and 3.6 months in the placebo group. There was similarly very little difference in the PSA response, with 2.6% in the sipuleucel-T group and 1.3% in the placebo group having at least a 50% reduction in PSA. A total of 65.2% of the patients had a grade 1 or 2 AE, most of which occurred within 1 day of the infusion. The most common AEs were chills, fever, headache, flu-like illness, myalgia, hypertension, hyperhidrosis, and groin pain (likely related to infusion catheter placement). Only 0.9% of patients were unable to get all 3 infusions as a result of infusion-related AEs.7
Sipuleucel-T has been relatively underutilized compared with initial projections, and only rarely does a patient respond to therapy. Underutilization may be due to the cumbersome nature of the administration, which requires 3 apheresis infusions in a month, the not-infrequent requirement for placement of an apheresis catheter, the low PSA response rate, or a combination of these and other factors. The exact reasons for low utilization of this agent is unclear.
Cabazitaxel
Cabazitaxel is a tubulin-binding taxane that was FDA-approved in 2010 for the treatment of men with mCRPC who progressed on docetaxel. Its efficacy was evaluated in the TROPIC trial,8 in which 755 patients with mCRPC who progressed on or after docetaxel were randomized to either cabazitaxel or to mitoxantrone. Both arms received prednisone 5 mg orally twice daily. The study met its primary endpoint of OS, with an improvement in median OS of 2.4 months. The most common toxicity was hematologic, with 82% of patients developing grade 3 or greater neutropenia and 8% developing febrile neutropenia. A total of 47% of patients developed diarrhea; 6% developed grade 3 or greater diarrhea. Grade 3 or greater peripheral neuropathy was reported in the cabazitaxel arm in 1% of patients, but 14% for all grades. Patients with grade 2 or greater peripheral neuropathy on entry were excluded from the study.8
Two trials are currently evaluating the use of cabazitaxel at 20 mg/m2 or 25 mg/m2. One of these trials utilizes this dosing schema in the post-docetaxel space (PROSELICA), and the other does so in the chemotherapy-naïve population, with a control group consisting of docetaxel every 3 weeks at 75 mg/m2 (FIRSTANA). As a result of the toxicities encountered with the 25-mg/m2 dosage and the fact that phase I studies suggested either a 20 mg/ m2 or 25 mg/m2 dosing schema,9 the FDA required the sponsor to conduct these trials. In the TROPIC trial, despite the relatively high rate of febrile neutropenia, prophylactic granulocyte colony-stimulating factor (G-CSF) was not allowed in the first cycle.8 In the FDA-approved package insert, a black box warning states: “Neutropenic deaths have been reported. Obtain frequent blood counts to monitor for neutropenia. Do not give JEVTANA if neutrophil counts are ≤1500 cells/mm3.” In addition, there is a statement that prophylactic G-CSF should be considered for patients with high-risk features (age >65 years, poor PS, previous episodes of febrile neutropenia, extensive prior radiation ports, poor nutritional status, or other serious comorbidities) that predispose patients to increased complications from prolonged neutropenia. Cabazitaxel is now 1 of 4 drugs approved after conducting phase III studies in patients with prior docetaxel treatment. These drugs also include abiraterone, enzalutamide, and radium-223.
Cabazitaxel today is the only drug relegated to the post-docetaxel space, as abiraterone, radium-223, and enzalutamide do not require (from a regulatory perspective) prior chemotherapy treatments. The use of cabazitaxel may or may not be superior to docetaxel in the frontline CRPC setting. The FIRSTANA study will address this issue, and likely report top-line results in 2015.
Abiraterone
Abiraterone is a very potent inhibitor of P450 c17 (CYP17), an enzyme that has 2 distinct activities: 17-20 lyase and 17-alpha hydroxylase. This enzymatic action is used in the conversion of pregnenolone and progesterone to 17-OH pregnenolone and 17-OH progesterone, and from there to dehydroepiandrosterone (DHEA) and androstenedione, which is the penultimate step in testosterone production. Abiraterone was approved by the FDA in 2011 for use in patients with mCRPC who have been previously treated with docetaxel. In 2012, this indication was expanded to include patients who were chemotherapy-naïve.
Two pivotal, randomized phase III trials demonstrated improvement in OS compared with placebo. In the COU-AA-301 trial,10 1195 patients previously treated with docetaxel were randomized to abiraterone plus prednisone or placebo plus prednisone. The primary endpoint of OS was met with an improvement in OS of 3.9 months compared with placebo. All secondary endpoints also favored abiraterone, with a PSA response rate of 29% vs 6%. The most common AE was fatigue, which was similar in both groups. There were more mineralocorticoid symptoms consisting of fluid retention in the abiraterone group, and more hypokalemia in the abiraterone group, although the majority of these side effects were grade 1 in nature.
In the COU-AA-302 trial,11 1088 chemotherapy-naïve patients were randomized to abiraterone plus prednisone or to placebo plus prednisone. The primary endpoints were OS and radiographic PFS. There was an 8.2-month improvement in radiographic PFS favoring abiraterone. The OS in in the initial evaluation did not pass the prespecified value for the interim analysis; however, the final OS results reveal an improvement in OS of 4.4 months.12Overall, this is a well-tolerated and effective medication. In both studies, the rate of discontinuation of the drug was about 20% in both the treatment and placebo arms. The initial COU-AA-302 trial was stopped at an interim analysis by the data monitoring committee (DMC), and at that time OS differences were not apparent between the arms. Only later did an OS difference emerge (which was a co-primary endpoint).
The dosing of abiraterone is subject to discussion by some. Consuming food can result in better absorption of abiraterone, and using smaller dosages could likely result in cost savings that are not currently being utilized.13 A single-institution retrospective analysis by Leibowitz-Amit and colleagues14 compared low-dose abiraterone with food to full-dose abiraterone after fasting, and found no difference in OS or PSA response rate, except in chemotherapy-naïve patients, where there was a trend to reduced PSA response rate in the lower-dose group. Considerable cost savings with respect to abiraterone could be obtained with either dose reduction or a generic drug. Patent protection may expire within the next several years. Should this occur, there are considerable implications for the mCRPC space, particularly with drugs that are currently priced far in excess of a generic abiraterone price.
Enzalutamide
Enzalutamide is an androgen receptor (AR) antagonist with a strong antagonist effect in binding the AR. This antagonism inhibits the DNA binding of the AR, and thereby inhibits cofactor recruitment that otherwise would have significant effects on transcription. Enzalutamide received FDA approval in 2012 for the treatment of patients with mCRPC who had been previously treated with a docetaxel-containing chemotherapy regimen; in 2014, this approval was extended to patients who were chemotherapy-naïve. Two large phase III trials have demonstrated the efficacy of enzalutamide in the treatment of patients with mCRPC.
In the AFFIRM trial,15 1199 patients who were previously treated with a docetaxel-containing chemotherapy regimen were randomized to enzalutamide or placebo. The primary endpoint of OS was met with an improvement in OS of 4.8 months favoring enzalutamide. There was also improvement in the secondary endpoints of radiographic PFS (8.3 months vs 2.9 months; HR, 0.40; P <.001) and the time to the first skeletal-related event (SRE; 16.7 months vs 13.3 months; HR, 0.69; P <.001).
In the PREVAIL trial,16 1717 patients with asymptomatic or minimally symptomatic mCRPC who had not received chemotherapy, abiraterone, or ketoconazole were randomized to enzalutamide or placebo. The 2 primary endpoints were radiographic PFS and OS. The median radiographic PFS had not been reached in the enzalutamide group and was 3.9 months in the placebo group, with an HR of 0.19 (P <.001). At 12 months, the radiographic PFS was 65% in the enzalutamide group versus 14% in the placebo group. The median OS was estimated at 32.4 months in the enzalutamide arm and 30 months in the placebo group, with an HR of 0.71 (P <.001).
The most common AEs in both trials were fatigue, hot flashes, and headache. In the AFFIRM trial, there were 5 seizures in the enzalutamide arm and none in the placebo arm. This has resulted in a warning against the use of enzalutamide in patients with a history of seizures. In the PREVAIL trial, only 1 seizure was noted in each arm; patients with a history of seizure or a condition that could confer a predisposition to seizure were specifically excluded.
Though generally well tolerated, some patients have experienced substantial fatigue. Given that many men currently receiving enzalutamide are in the pre-docetaxel space and are otherwise asymptomatic, drug-induced fatigue may be the only symptom in some patients receiving this drug. No prospective trials have been conducted head to head against abiraterone/ prednisone to date. The price is considered high by many, and many countries cannot afford to use this new drug. This is an issue common to all of the new life-prolonging agents.
Radium-223
Radium-223 is a bone-targeted, alpha-emitting radiopharmaceutical that was FDA-approved in 2013 for the treatment of patients with mCRPC. Radium (Ra), like calcium (Ca), strontium (Sr), and barium (Ba), is an alkaline earth metal in the periodic table of the elements, which, as a family, localize to areas of osteoblastic metastasis. The FDA approval came from the results of ALSYMPCA,17 a phase III trial in which 921 patients received radium-223 or placebo, and all patients received SOC. SOC was confined to nonchemotherapeutics and older hormonal therapies such as flutamide, bicalutamide, and dexamethasone. Enzalutamide and abiraterone were not yet approved when the phase III study was performed. Patients received a total of 6 injections, each of which was administered at 4-week intervals at a dosage of 50kBq/kg. The becquerel (Bq) is an SI unit for radioactivity defined as the activity of a quantity of radioactive material in which 1 nucleus decays per second.
The primary endpoint was OS, with secondary endpoints including time to first symptomatic skeletal event (SSE). No radiographic monitoring was proscribed as a part of the trial. The patients included in the trial had to have mCRPC, at least 2 bone metastases as detected on bone scan, no visceral metastasis, and no lymph nodes in excess of 3 cm. Patients must also have had symptomatic disease, as defined as regular use of any analgesic medications or radiation therapy for bone pain in the preceding 12 weeks; 55% of the patients were taking opioids for palliation of pain. Patients were required to have docetaxel pretreatment, refuse docetaxel, be considered unfit for docetaxel, or not have docetaxel available.17
The trial met both its primary and secondary endpoints at an interim analysis, and the trial was stopped by the DMC as a consequence of the prespecified statistical analysis plan. There was an OS advantage of 3.6 months (14.9 months for radium-223 and 11.3 months for placebo), with an HR of 0.70 (P <.001). Time to first SSE was 15.6 months for radium-223 and 9.8 months for placebo, giving an almost 6-month advantage with an HR of 0.66 (P <.001).17 However, the analysis was not performed with an endpoint of SSE or death, which are preferred by the FDA.
There were fewer total AEs in the radium-223 arm (93%) compared with the placebo arm (96%), including fewer people stopping the medication as a result of an AE (16% vs 21%). There were more thrombocytopenia and neutropenia in the radium-223 arm. However, a subgroup analysis of disease burden revealed that patients with fewer than 6 lesions on bone scan did not do better than the placebo group, with an HR for OS of 0.95 (95% CI, 0.46-1.95), while patients with 6 to 20 lesions had an HR for OS of 0.71 (95% CI, 0.54-0.92), and those with >20 lesions had an HR for OS of 0.64 (95% CI, 0.47-0.88).17
There is not a large PSA response in patients treated with radium-223, as seen in only 14% of patients in the radium-223 group who had a 30% or greater reduction in PSA 4 weeks after the last injection compared with 4% in the placebo arm. The time to PSA rise was statistically significant (P <.001), but clinically not significant: 3.6 months in the radium-223 arm and 3.4 months in the placebo arm. Of note, in the subset analysis looking at previous docetaxel exposure, the HR for OS in chemotherapy-naïve patients was 0.74 (95% CI, 0.56-0.99) and the HR for chemotherapy-experienced patients was 0.71 (95% CI, 0.56-0.89). In neither case did the 95% CIs overlap 1.0.17 The FDA approved radium-223 without regard to prior docetaxel use.
Controversies in current radium-223 use include the fact that those individuals with no prior docetaxel use had refused docetaxel or were considered unfit for it. These data were not captured in the ALSYMPCA case report form, and some investigators have contended that radium-223 use in the pre-docetaxel space is controversial. Although there are limited data to suggest that docetaxel post-radium-223 is safe, no prospective trials have demonstrated this to be the case. There is also the question of safety/efficacy when radium-223 is used in combination with abiraterone or enzalutamide. Initial reports18 indicate no safety concerns, but the question of efficacy awaits prospective randomized trials. Considering exactly how radium-223 fits into the current treatment paradigm is controversial, and no radiopharmaceutical has achieved widespread use. Administration of radium-223 is restricted to nuclear medicine physicians and selected properly licensed radiation oncologists. These physicians are referral specialists, and rarely do they make treatment decisions in the complex current mCRPC landscape.
Denosumab
Men on long-term ADT are at increased risk for SREs, which are typically defined as pathologic fracture, need for radiation or surgery to bone, and spinal cord compression. The use of zoledronic acid given monthly at a dosage of 4 mg IV has been shown to reduce the rate of developing SREs by 25%; however, the treatment had no effect on OS.19 Denosumab is a monoclonal antibody that binds to RANKL, which is a mediator of osteoclast formation. In a subsequent randomized trial, 1904 men were randomized to either denosumab 120 mg monthly or zoledronic acid 4 mg every 3 weeks.20 The primary endpoint was the time to first on-study SRE, which was assessed for noninferiority and for superiority as a secondary endpoint. Denosumab demonstrated a benefit of 20.7 months compared with 17.1 months for zoledronic acid (HR, 0.82; P = .0002 for noninferiority and P =.008 for superiority). Any AE was similar in both groups, but there were more grade 3-4 AEs in the denosumab arm (66% vs 72%; P = .01). More hypocalcemia was seen in the denosumab arm (6% vs 13%; P <.0001). The rate of osteonecrosis of the jaw (ONJ) was similar in both groups.20 That said, it was not clear that denosumab would add additional value to the use of newer agents such as radium-223, enzalutamide, or abiraterone. In each case, these agents have independently been shown to reduce rates of SREs.
In patients with nonmetastatic CRPC, denosumab was shown to slightly delay the development of metastatic bone disease. In a large phase III trial, 1432 patients were randomized 1:1 to either denosumab 120 mg every 4 weeks or placebo.21 Bone metastasis-free survival was greater in the denosumab group, with a median duration of 29.5 months versus 25.2 months for placebo (HR, 0.85; P = .028). There was no survival advantage, however, with a median OS of 43.9 months for denosumab and 44.8 months for placebo (HR, 1.01; P = .91). The only difference in AEs was in ONJ, which occurred in 5% of patients taking denosumab versus none in the placebo group, and hypocalcemia, which occurred in 2% in the denosumab arm versus less than 1% in the placebo group. The FDA reviewed this trial and did not issue a label for this indication. Thus, denosumab in the nonmetastatic disease setting is not considered to be SOC.
It is not completely clear how much an agent such as denosumab will contribute if patients are under treatment with newer agents such as abiraterone or enzalutamide. Denosumab does not prolong survival. Trials have not addressed this question.
Sequencing of Agents in mCRPC
The past 5 years have witnessed an explosion of new therapies in the treatment of mCRPC, with 5 new agents gaining FDA approval for an improvement in OS. While exciting and certainly good for patients, these approvals have generated much discussion and debate about what the best agent is and when it should be used. As docetaxel was first approved in 2004, and it took 6 years for another agent to demonstrate that it improved OS, it was certainly natural that the next step was to look for an agent that could be used when docetaxel fails. This set up the schema of the post-docetaxel and pre-docetaxel spaces, although now this segmentation into pre- and post-docetaxel is blurring as 3 agents (radium-223, abiraterone, and enzalutamide) have now shown a survival benefit both prior to chemotherapy and after docetaxel.
No one sequence can be endorsed over any other at this time. Trials will never address all of the alternatives, nor should they, given the huge number of potential options. It is not clear how long the field will remain in a sequencing paradigm, and the need for evidence for combination therapies and better patient selection seem to be higher priorities. Treating patients with multiple agents seems intuitively correct, but the data are not present to support a strong view.
Cross-Resistance
With the use of single agents in sequence, evidence is emerging that cross-resistance can be conferred by one agent to the next, and that responses may diminish after the use of multiple therapies. Although formal studies are lacking, the retrospective data with abiraterone and enzalutamide in sequence are compelling. With enzalutamide, for example, we see a PSA response rate of 78% in the pre-docetaxel setting and a response rate of 54% in the post-docetaxel setting, whereas retrospective studies have shown a response rate of 23% to 39% post-docetaxel, post-abiraterone.22-24 This is similar to the response rate of 62% seen with abiraterone in the pre-docetaxel setting and a PSA response rate of 38% (29% confirmed) in the post-docetaxel setting. Retrospective studies have shown a response rate for abiraterone of 3% to 8% in the post-docetaxel, post-enzalutamide setting25-26 and of 17% in the post-docetaxel, post-cabazitaxel setting.27 Taken together, there is clear evidence for cross-resistance between enzalutamide and abiraterone when these drugs are used sequentially.
Optimizing Patient Selection
None of these newer agents have been compared with one another; the newer therapies to date are compared with placebo, prednisone, mitoxantrone, or best SoC. No level 1 evidence will be available for treatment choices until there are head-to-head trials. Until then, clinicians will speculate and discuss, but no one will truly know what course of action might be best for a patient. Predictive biomarkers would be tremendously helpful in this regard, though none are currently accepted in general clinical practice.
Currently, the evaluation for the initial treatment of mCRPC focuses on 2 aspects. The first is the disease, and the important issues here include: the pace of the disease (PSA doubling time); the location of the disease (visceral involvement, bone involvement); the burden of disease (larger vs smaller extent of metastasis); and degree of symptomatology. The second evaluation regards the patient, which includes the patient’s PS, various laboratories related to organ/marrow function, willingness to undergo chemotherapy, and willingness or ability to tolerate the financial toxicity associated with some of the treatments.
In a patient with no visceral disease and a relatively slowly rising PSA and who is asymptomatic or minimally symptomatic, initial therapy with sipuleucel-T is reasonable. Thus, both low pace and low burden of disease might be considered in the treatment selection. The therapy can be given in 4 weeks’ time, and then the patient can move to other therapies. Two phase II studies are evaluating the use of sipuleucel-T and either abiraterone or enzalutamide in combination or in sequence.28-29 Both abiraterone and enzalutamide appear to be safe when given simultaneously, but whether efficacy will be affected has yet to be determined.
If a patient has bone-only disease and is symptomatic, radium-223 is a reasonable approach. The benefit for radium-223 was best appreciated in patients with 6 or more metastatic bone lesions. A patient with a single bone lesion may or may not achieve maximum benefit from radium-223. In clinical trials, only 16% of patients had a 30% decline in PSA, so the tempo of the disease is also important to consider. Unlike sipuleucel-T, a full course of radium-223 is given over 6 months, so it is critical to select patients whose disease will allow a 6-month “runway” to complete the therapy. Two phase III trials are currently under way evaluating the combination of radium-223 with abiraterone and enzalutamide (Table 2).
The other special case would be the situation in which a patient has visceral involvement. Here, the level 1 evidence would support enzalutamide or docetaxel. The COU-302 trial excluded patients with visceral involvement, so abiraterone has not been formally tested in the pre-docetaxel setting with visceral disease. However, clear evidence of activity for abiraterone in patients with visceral disease is seen in the post-docetaxel space.
Selected Potential Predictive Biomarkers
Resistance to abiraterone and enzalutamide was reported as a result of a variant of the AR, specifically the isoform encoded by the AR splice variant 7 (AR-V7).30 This transcript encodes for a cryptic exon associated with a premature stop codon, thereby deleting the C-terminal portion of the AR. This truncated AR can serve as a ligand-independent transcription factor, and is capable of stimulating expression of a series of genes associated with cellular proliferation. Table 3 lists predictive biomarkers. Antonarakis et al30 evaluated the impact of this variant on resistance to newer hormonal therapy. The study used an assay that utilized circulating tumor cells (CTCs) isolated by immunomagnetic beads and a RT-PCR assay specifically designed to detect AR-V7 mRNA.Of the 62 patients evaluated, 31 received enzalutamide and 31 received abiraterone. A total of 39% of the enzalutamide-treated patients and 19% of the abiraterone-treated patients had detectable AR-V7. In the patients receiving enzalutamide, the PSA response rate among the AR-V7-positive patients was 0% versus 53% among AR-V7-negative patients. In the patients receiving abiraterone, the PSA response rate among the AR-V7-positive patients was 0% versus 68% in the AR-V7-negative population. In a follow-up study separately reported, Antonarakis et al31 evaluated taxane-treated patients who were prospectively enrolled and evaluated for the AR-V7 isoform via CTCs. The PSA response in both AR-V7-negative and -positive patients was not statistically significant, nor was the median PFS.31 Taken together, these studies indicate that the AR-V7 mutation induces resistance to enzalutamide and abiraterone, but not to chemotherapy with docetaxel.
Cell-free DNA collected from peripheral blood is an area of active investigation. Azad et al32 collected tumor cell-free DNA from the serum of 53 patients who were starting therapy with enzalutamide. They showed that having AR amplification/gain was more common in patients progressing on enzalutamide compared with abiraterone or other agents (53% vs 17% vs 21%, respectively; P = .02). In addition, patients who also had an AR copy number gain and/or an exon 8 mutation had a lower response rate to enzalutamide (P = .013) and had a shorter median PFS (4.6 months vs 2.3 months; P = .01). These data suggest that cell-free DNA may serve as a predictive biomarker for patients with mCRPC being treated with enzalutamide.
An AR-V7 detection methodology that uses a monoclonal antibody to a unique AR-V7 epitope was presented at the 2015 annual meeting of the American Association for Cancer Research.33 The methodology utilized this antibody in CTCs evaluated by the Epic Sciences CTC detection platform. The expression of V7 was associated with a high rate of resistance to new responded. hormonal agents, but some docetaxel-treated patients responded.
Another recent report yet to appear in peer-reviewed format involves AR-V7 detection using RNA isolation followed by RT-PCR without CTC isolation.34 Such an assay has obvious advantages in that it avoids the CTC isolation step, which can complicate assessments and requires immunomagnetic cell-based CTC assays. Preliminary work suggests that patients resistant to newer-generation hormonal agents commonly express AR-V7, but prospective assessments on these assays have yet to be performed.
Olaparib acts as an inhibitor of the enzyme poly(ADP-ribose) polymerase (PARP),which functions as a DNA repair enzyme.
It is currently FDA-approved for the treatment of patients with BRCA-positive, advanced ovarian cancer who have been treated with 3 or more prior lines of chemotherapy.
The TOPARP study35 evaluated the efficacy of olaparib in 50 patients with mCRPC who had failed previous docetaxel therapy. In this study, presented only in abstract form, 96% had also received abiraterone and 58% received cabazitaxel. Of the 49 evaluable patients, 16 had a response to the olaparib therapy, defined as a greater than 50% decline in PSA. The investigators then performed DNA sequencing of the patients’ tumors to identify a possible genetic biomarker. Mutations in DNA repair genes were found in 15 of the 49 evaluable patients (30.6%) enrolled in the study. Of the 15 patients with mutations, 13 of them responded to olaparib, with 7 of 7 patients with a BRCA2 mutation responding to olaparib. DNA-repair defects may be a powerful biomarker for olaparib action, and these defects may be more common than previously appreciated in patients with mCRPC. A larger trial is now being planned.
Selected New Agents
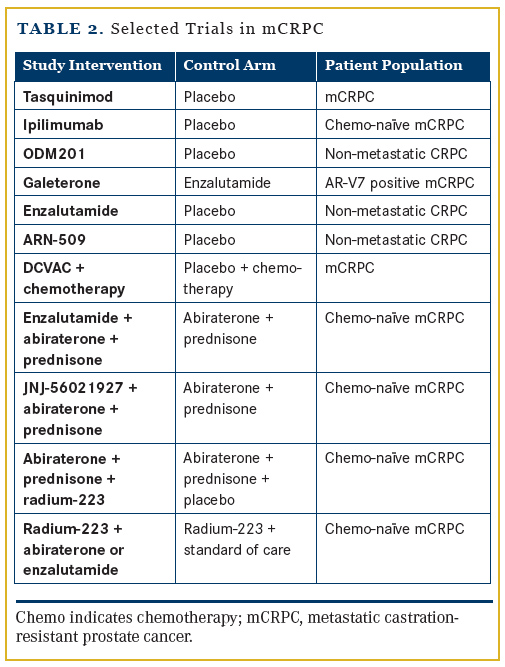
There are now myriad options for the treatment of patients with mCRPC, and more are being studied (Table 2). ARN509 is an AR inhibitor; galeterone is an AR inhibitor that also has activity as an inhibitor of CYP17 and downregulates AR variants in preclinical studies. PROSTVAC-VF/TRICOM and DCVAC/ PCa are vaccine therapies in clinical trials. ODM201 is an AR inhibitor, and tasquinimod is a small-molecule inhibitor that alters the tumor microenvironment. Ipilimumab, FDA-approved for melanoma, is also being tested in advanced prostate cancer.
Synopsis
Right now, there is the enviable problem of having many therapies available to our patients, but a lack of level 1 evidence comparing these new agents to one another. In the future, commercial assays for predictive biomarkers will likely be available to help guide therapeutic decisions (much like cetuximab for KRAS wild-type colon cancer or erlotinib for EGFR-positive non-small cell lung cancer). Imatinib was FDA-approved for Bcr-Abl-translocated leukemia in 2001, marking the beginning of personalized cancer therapy. Now 14 years after that milestone, progress in the area of personalized prostate cancer therapy is being made, but much more progress is needed before the survival curves bend in a more meaningful manner.
Affiliations: Brian Lewis, MD, MPH, is assistant professor of Clinical Medicine, and Oliver Sartor, MD, is Laborde Professor for Cancer Research, Departments of Medicine and Urology, Tulane University School of Medicine, New Orleans, LA.
Disclosures: Dr Lewis reports no relevant financial conflicts of interest to disclose. Dr Sartor has been a consultant or advisory board member for Bayer, Algeta, Sanofi, Bellicum Pharmaceuticals, and Dendreon; he has received grant support from Bayer, Sanofi, Janssen, and Dendreon.
Address correspondence to: Oliver Sartor, MD, Box SL-42, 1430 Tulane Ave, New Orleans, LA 70112. Phone: 504-355-7970; fax: 504-988-1813; email: [email protected].
References
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502-1512.
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513-1520.
- Sweeney C, Chen Y, Carducci M, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): an ECOG-led phase III randomized trial. J Clin Oncol. 2014;32:5s(suppl; abstr LBA2).
- Gravis G1, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomized, open-label, phase 3 trial. Lancet Oncol. 2013;14(2):149-158.
- Medical Press. Adding chemotherapy to standard prostate cancer treatment may extend life expectancy. http:// medicalxpress.com/news/2015-05-adding-chemotherapy-standard-prostate-cancer.html. Accessed May 14, 2015.
- Kellokumpu-Lehtinen PL, Harmenberg U, Joensuu T, et al. 2-weekly versus 3-weekly docetaxel to treat castration-resistant advanced prostate cancer: a randomized, phase 3 trial. Lancet Oncol. 2013;14(2):117-124.
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-422.
- De Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomized open-label trial. Lancet. 2010;376:1147-1154.
- Mita AC, Denis LJ, Rowinsky EK, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15:723-730.
- De Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138-148.
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152-160.
- Ryan C, Rosenberg J, Lin A, et al. Effect of concomitant food intake on pharmacokinetics of abiraterone acetate, a 17 α hydroxylase C17,20-lyase inhibitor in castration-resistant prostate cancer (CRPC). Presented at: AACR-NCI-EORTC International Conference; October 22-26, 2007; San Francisco, CA. Abstract C2
- Leibowitz-Amit R, Atenafu E, Seah J, et al. Low-dose abiraterone (abi) with food in men with metastatic castration-resistant prostate cancer (mCRPC): the Princess Margaret Cancer Centre experience. J Clin Oncol. 2014;32(suppl 5s; abstr 5077).
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424-433.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213-223.
- Dan TD, Eldredge-Hindy HB, Hoffman-Censits J, et al. Hematologic toxicity of concurrent administration of radium-223 and next-generation antiandrogen therapies [published online February 25, 2015]. Am J Clin Oncol.
- Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458-1468.
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomized, double-blind study. Lancet. 2011;377:813-822.
- Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomized, placebo-controlled trial. Lancet. 2012;379:39-46.
- Thomson D, Charnley N, Parikhet O. Enzalutamide after failure of docetaxel and abiraterone in metastatic castrate resistant prostate cancer: results from an expanded access program. J Clin Oncol. 2014;32(suppl 4; abstr 188).
- Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2014;65:30-36.
- Bianchini D, Lorente D, Rodriguez-Vida A, et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer pretreated with docetaxel and abiraterone. Eur J Cancer. 2014;50:78-84.
- Loriot Y, Bianchini D, Ileana E, et al. Antitumor activity of abiraterone acetate against metastatic castration-resistant prostate cancer prgressing after docetaxel and enzalutamide. Ann Oncol. 2013;24:1807-1812.
- Noonan KL, North S, Bitting RL, et al. Clinical activity of abiraterone in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24:1802-1807.
- Wissing MD, Coenen JL, Van den Berg P, et al. CAST: a retrospective analysis of cabazitaxel and abiraterone acetate sequential treatment in patients with metastatic castrate-resistant prostate cancer previously treated with docetaxel. Int J Cancer. 2015;136:E760-E762.
- Petrylak D, Quinn DI, Dreicer R, et al. 774P - STRIDE, a randomized, phase 2, open-label study of sipuleucel-T with concurrent vs sequential enzalutamide in metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. 2014;25(suppl_4):iv255-iv279. doi:10.1093/annonc/mdu336.
- Small E, Lance R, Redfernet C, et al. A randomized phase II trial of sipuleucel-T with concurrent or sequential abiraterone acetate (AA) plus prednisone (P) in metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol. 2013;31(suppl; abstr 5047).
- Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028-1038.
- Antonarakis ES, Lu C, Chen Y, et al. AR splice variant 7 (AR-V7) and response to taxanes in men with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2015;33(suppl 7; abstr 138).
- Azad A, Wyatt A, Volik S, et al. Genomic analysis of circulating cell-free DNA (cfDNA) to investigate mechanisms of resistance to enzalutamide (ENZ) in metastatic castration-resistant prostate cancer (mCRPC) patients (pts). J Clin Oncol. 2015;33(suppl 7; abstr 157).
- Kelvin J, Lu D, Packer D, et al. Single cell analysis of AR N terminal, AR C terminal and the ARV7 splice variant in the CTCs of metastatic castration-resistant prostate cancer (mCRPC) patients. Presented at: 106th Annual Meeting of the American Association for Cancer Research; April 18-22, 2015; Philadelphia, PA. Abstract 1588.
- Liu X, Ledet E, Qi Y, et al. A novel blood-based assay for detecting androgen receptor splice variants in patients with advanced prostate cancer. Presented at: 21st Annual PCF Scientific Retreat; October 23-25, 2014; Carlsbad, CA.
- Mateo1 J, Sandhu1 S, Miranda S, et al. DNA repair defects and antitumor activity with PARP inhibition: TOPARP, a phase II trial of olaparib in metastatic castration-resistant prostate cancer. Presented at: 106th Annual Meeting of the American Association for Cancer Research; April 18-22, 2015; Philadelphia, PA. Abstract CT322.