Introduction
Multiple myeloma (MM), characterized by a malignant proliferation of monoclonal plasma cells,1-4 comprises clonally diverse subsets of malignant plasma cells exhibiting a vast genetic diversity that contributes to the complex pathogenesis of this disease and underpins its difficulty in treatment.1-4 It is the second most common hematologic cancer in the United States: approximately 95,688 individuals have MM5 and 0.7% will be diagnosed during their lifetime.6 In the United States, it is estimated that 33,330 new cases of MM are diagnosed each year will be diagnosed in 2016,5 with 86,000 new cases worldwide.7 Overall, MM is more common in men than in women and twice as likely in African Americans compared with Caucasians.5
An MM diagnosis is based on the presence of a bone marrow clonal plasma cell count ≥10% and the presence of a monoclonal or M-protein.8,9 Recommended diagnostics include a detailed medical history, physical exam, routine laboratory testing, bone marrow biopsy/aspiration for cytogenetic analysis or fluorescence in situ hybridization (FISH), and radiographic imaging. Also recommended to further evaluate symptoms are magnetic resonance imaging (MRI), computed tomography (CT), or positron emission tomography (PET).8,9
In 2015, MM resulted in 11,240 deaths in the United States,10 a number that was projected to increase to 12,650 in 2016.5 Novel and targeted agents have improved survival, but patients with stable disease still experience symptoms, such as pain and fatigue, and report poor physical functioning.11 Longitudinal research demonstrated that patients with MM report significantly lower health-related quality of life than age- and gender-matched controls.12 MM also poses a financial burden on patients via increased work disability rates and high out-of-pocket treatment costs, among other factors.13
MM remains, for many patients, incurable.14 Yet, the survival and complete response rates for patients have improved with available therapies.15 Recent advances in genetic sequencing, bio-informatics, and clinical trial design have enabled a new era of precision medicine powered by the wider availability of genomic and clinical data.16-18 Precision medicine is now accelerating the discovery of novel targeted therapeutic agents by providing greater understanding of the genomic basis of cancers,16-18 including in MM.19
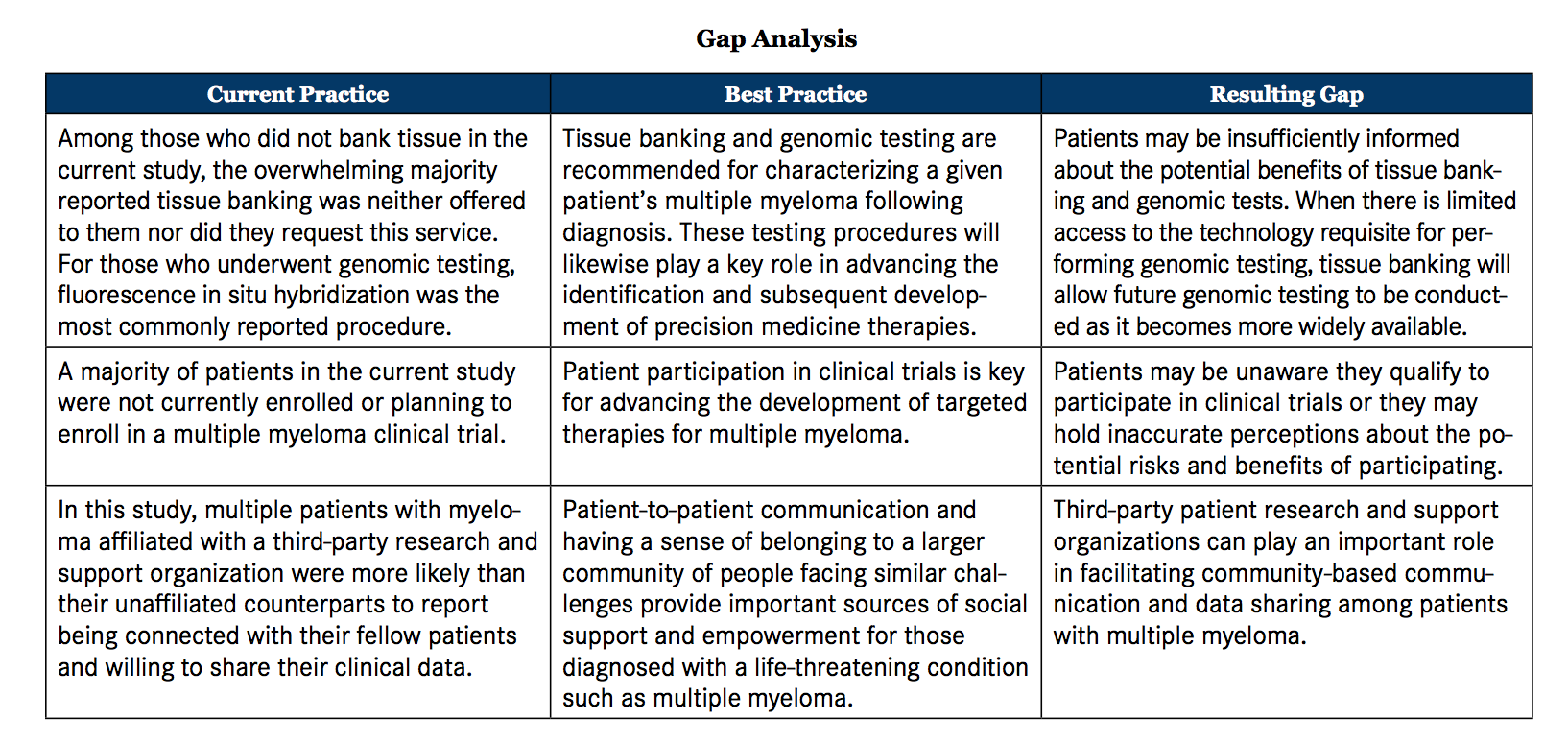
Research suggests better health outcomes result when patients are knowledgeable about their respective disease and feel self-empowered to make informed treatment decisions.20 However, prior research has shown that a minority of patients with lymphoma and/or MM reported having insufficient information about their disease.21 Third-party organizations focused on providing knowledge and support systems to patients with MM can potentially serve a meaningful role in filling this unmet need. Yet, the extent to which involvement with such organizations relates to patients’ understanding of their disease and to engagement in their diagnosis and treatment has not been investigated (see Gap Analysis).
Research Objectives
With the emerging importance of precision medicine, patients must play an active role in the diagnosis, treatment, and management of their disease. The current study assessed how, shortly after diagnosis, patients with MM perceive and understand 10 key decision points pertinent to diagnosis and treatment: provider choice, insurance coverage, diagnostic testing, imaging, tissue banking, genomics, connecting with other patients, standard of care, clinical trials, and sharing data. Additionally, this study examined if involvement with a third-party organization relates both to patients’ better awareness of, and engagement in, these critical decisions.
Methods and Materials
Participants
Patients with newly diagnosed MM were recruited by Kantar Health (New York, NY) using Multiple Myeloma Research Foundation (MMRF) 22 resources (n = 100) or from Lightspeed Research and its affiliated online panels (n = 77). Inclusion criteria for all participants were: lives in the United States, ≥25 years, diagnosed with MM in the past 4 to 12 months, self-reported being at least somewhat knowledgeable about MM, and self-reported having at least some input in treatment decisions.
Patient Survey
This study was conducted by Kantar Health and sponsored by the MMRF, with data fielded from April through September 2015. The study protocol was granted an exemption by the Pearl Institutional Review Board (Indianapolis, IN). Potential respondents were e-mailed an invitation to complete a 30-minute online survey. Participants indicated their informed consent by reading the e-mail invitation and then clicking the link to begin the survey.
As part of the survey, patient demographics were collected (Table 1). The 10 key decision points developed for the survey were based on results from an initial qualitative study with 26 patients with MM (recruited from Lightspeed Research and affiliate panels) and 6 hematologists/oncologists from a MedQuery online panel (unpublished data). To quantitatively evaluate these decision points, patients in the present study were asked about their experiences with provider choice, insurance coverage, diagnostic testing, imaging, tissue banking, genomics, connecting with other patients, standard of care, clinical trials, and sharing data.
Analyses
Descriptive statistics were calculated. Differences between MMRF- affiliated and nonaffiliated patients were assessed using column proportions tests. Specifically, the proportion of MMRF-affiliated patients, relative to nonaffiliated patients, was compared for each response category for each survey item. Due to low base rates, responses were collapsed across categories for some of these comparisons. There were no missing data, as the survey required responses to all items. Two tailed P-values (<.05) were considered statistically significant.
Results
Participant Characteristics
The study shows that 58% (n = 103) of respondents were male and 87% (n = 154) self-identified as Caucasian. MMRF-affiliated patients were significantly more likely to be Caucasian than nonaffiliated patients, whereas the latter were significantly more likely to self-identify as African American than the former. On average, nonaffiliated patients were older (56.78 years; standard deviation [SD] = 1.22) than MMRF-affiliated patients (52.92 years; SD = 1.09; P = .02). MMRF-affiliated patients were significantly more likely to report completing postgraduate work/graduate degree and being employed. Conversely, unaffiliated patients were significantly more likely to report having a high school degree or less or some college and being unemployed. MMRF-affiliated patients were significantly more likely to have Medicare coverage alone than nonaffiliated patients. Patient groups did not differ on the other demographic variables assessed.
Provider Choice
Compared with nonaffiliated patients, a significantly greater proportion of MMRF-affiliated patients reported seeking a second opinion after receiving their initial diagnosis (Table 2). When choosing a hematologist/oncologist, MMRF-affiliated patients were more likely to use disease-related criteria, including choosing a physician who is recognized as a clinical expert in the field, is published in this field, has a higher number of patients with MM in his/her practice, and whose practice is in geographic proximity. In contrast, nonaffiliated patients were significantly more likely to select a hematologist/oncologist from a recommendation or referral from another healthcare provider or because this specialist’s services were covered by insurance. MMRF-affiliated patients were significantly more likely than nonaffiliated patients to “agree strongly” or “very strongly” agree when asked if they feel comfortable phoning or e-mailing to ask their hematologist/oncologist questions and if they feel comfortable challenging when disagreeing or not understanding something (Table 2).
Insurance Coverage
Across patient groups, a minority reported difficulties gaining access to certain diagnostic tests and treatments due to insurance/reimbursement barriers (Table 2). MMRF-affiliated patients were more likely to report insurance problems than nonaffiliated patients, a difference that was not statistically significant.
Diagnostic Testing
MMRF-affiliated patients were significantly more likely than nonaffiliated patients to report understanding their diagnostic test results “very well” or “extremely well” (Table 2) and were significantly more likely to view them- selves as “very well” or “extremely well” informed about their disease com- pared with nonaffiliated patients. Unaffiliated patients were significantly more likely to be unaware or unsure of their MM and light chain types. A significantly greater proportion of MMRF-affiliated patients reported discussing their risk profile with their hematologist/oncologist (Table 2).
Imaging
Most patients in both groups reported receiving x-rays, although the more sensitive advanced diagnostic imaging, such as MRI, CT scans, or PET scans, were less frequently reported (Table 2). MMRF-affiliated patients were significantly more likely than nonaffiliated patients to have requested a PET scan if they had not received this procedure, whereas a significantly larger percentage of nonaffiliated patients reported they planned to have a PET scan in the near future if they had not already done so. Patients’ most frequently cited reasons for not having a PET scan were their doctor had not mentioned it, followed by not understanding the need for this procedure (Table 2).
Tissue Banking
A significantly larger proportion of MMRF-affiliated patients were willing to bank tissue, and to report having actually done so, for research purposes via a bone marrow biopsy compared with nonaffiliated patients (Table 2). Nonetheless, sizeable percentages of patients in both groups have not engaged in this practice. Among those who did not bank tissue, the majority reported that tissue banking was neither offered to them nor did they request this service (Table 2).
Genomics
MMRF-affiliated patients were significantly more likely than MMRF- affiliated patients to have reported undergoing genomic testing (Table 3) and were significantly more likely than to report understanding their results “very well” or “extremely well.” A minority of patients in each group reported understanding their genomic test results “not very well” or “not well at all.” MMRF-affiliated patients were more likely to report fluorescence in situ hybridization (FISH), cytogenetics/karyo- typing, and gene expression profiling, whereas the 2 patient groups were comparably likely to report genomic sequencing and that FISH was the most common type of genomic test they underwent. Overall, MMRF-affiliated patients were significantly more likely than nonaffiliated patients to know if they had a genetic abnormality. Of those patients who had not undergone testing, a majority of both groups reported that their physician had not mentioned the need for genomic testing (Table 3). Of relevance to genomic testing, patients were asked if they had heard of “precision medicine,” and the majority were unfamiliar with this term.
Connecting with Other Patients
MMRF-affiliated patients were significantly more likely than nonaffiliated patients to report being connected with other patients (Table 3) and through different online venues, including Facebook, e-mail, other social media platforms, or online forums. Conversely, nonaffiliated patients were significantly more likely to report using in-person support groups for this purpose (Table 3).
Standard of Care
For both groups, over half of patients were unaware or unsure of the guideline-recommended standard of care for MM treatment (Table 3). Among those who were aware, MMRF-affiliated patients reported greater awareness than unaffiliated patients, but this difference was statistically nonsignificant. Both MMRF-affiliated and nonaffiliated patients were similarly likely to report being “very involved” or “extremely involved” in their treatment decisions (Table 3).
Clinical Trials
MMRF-affiliated patients were significantly more likely to report either participating in or planning to participate in clinical trials, compared with nonaffiliated patients (Table 3). However, a majority in both groups were not currently planning to enroll in an MM clinical trial. Of these individuals, MMRF-affiliated patients were more likely to report actively looking into clinical trial information than nonaffiliated patients, but this difference was not significant. Conversely, nonaffiliated patients were significantly more likely to report being “very willing” to participate in a clinical trial. The most common reasons cited by patients for nonparticipation were satisfaction with current treatment, more often by MMRF-affiliated patients; not knowing enough about clinical trials; and concerns surrounding the safety and side effects of experimental therapies (Table 3).
Sharing Data
MMRF-affiliated patients were significantly more likely to indicate their willingness to share their health information to advance MM research. Additionally, MMRF-affiliated patients were more likely than nonaffiliated patients to use a mobile app or spreadsheet to track and share their test results (Table 3).
Discussion
Due to the heterogeneity of MM and its complex pathogenesis, drug discovery is now being driven by the development of precision medicine-based molecular targeting strategies.16-18 The Precision Medicine Initiative is accelerating the discovery of novel targeted therapeutic agents for patients with MM, with the goal of swiftly translating cutting-edge research into available next-generation treatments.16-18 When initially receiving an MM diagnosis, patients are challenged with a chaotic influx of information regarding a life-threatening cancer, various diagnostic test results, new encounters with their hematologist/oncologist, and impending treatment decisions. This study assessed how individuals with MM experience key decision points in their patient journey following initial diagnosis through treatment. Involvement with a third-party research and support organization, in this case the MMRF, may facilitate patients’ understanding of these issues. However, gaps were also identified regarding knowledge of the standard of care, awareness of precision medicine, understanding of and participation in genomics research, and involvement in clinical trials.
The present research characterized some of the key informational needs specific to patients early in their journey, particularly those impacted by their involvement with a third-party organization. MMRF-affiliated patients, compared with unaffiliated patients, were significantly more informed on their MM disease state and reported a greater understanding of their various diagnostic test results. They were also better prepared for early, critical discussions with their hematologist/oncologist regarding their diagnosis and treatment-related decisions. This is especially notable because many of the initial decisions that a patient makes when under- going diagnostic testing have considerable downstream ramifications on their prognosis, individualized treatment options, and availability of pre- treatment biologic samples. These findings have important implications, as prior research has shown greater health literacy among patients with cancer can be traced to a higher likelihood of receiving treatment.23
Patient activation, which occurs when patients have adequate knowledge about their disease and the ability to act upon this information, is linked to better health behaviors and improvements in a variety of health outcomes.24 Aligned with this prior research, patients in both groups reported comparable levels of engagement in their treatment decisions. However, MMRF-affiliated patients appeared to play a more active role in choosing an appropriate provider, as they were more inclined to use disease-related criteria when choosing their hematologist/oncologist and were more than twice as likely to obtain a second opinion after initial diagnosis. This suggests these patients were more effectively able to self-advocate regarding their diagnosis and treatment, although further research will be needed to tie these behaviors to actual treatment outcomes.
The current study identified additional opportunities to provide support and information for patients with MM. As the results of a previous study demonstrate, the health-related information preferences of patients with cancer change over the course of their disease,25 so ongoing efforts are needed to understand the progress of the journey of patients who have MM. One key knowledge gap was the general lack of awareness regarding the standard of care for MM. Due to significant progress in the approval of effective therapeutic agents in recent years, efforts are needed to effectively inform patients and providers and to offer the requisite tools for them to select optimal treatments. Other important elements include the use of tissue banking and appropriate genomic testing. Overall, these findings underscore the need to close the gap between patients’ willingness to bank their tissue and following through with using this service. Continual efforts to improve all patients’ awareness of genomic testing are thus war- ranted, as this initiative is specifically working toward the collection and translation of genomic data into new precision-based therapies for MM.19
The availability of individualized information should lead to improved decision making for patients regarding treatments. However, some attention should be given to the existing barriers to care. According to the results of this study, work is still needed to ensure that all patients, regardless of their insurance coverage status or ability to pay, have access to the necessary diagnostic tests and treatments. Additionally, although there do appear to be strong connections within the patient community and a general willingness to share healthcare information for research purposes, only a small percentage of patients in each group were either participating in or planning to participate in clinical trials. With a rare disease like MM, encouraging a sufficient number of patients to enroll in clinical trials will be integral to identifying more precise, efficacious treatments and developing a cure. Adequate outlets for clinical trial enrollment and data sharing need to be provided to patients as they begin to understand the importance of taking an active role in their disease management.
Representing a substantial strength of the current study, the critical decision points assessed in this study were previously identified by patients with MM as both relevant and important through a qualitative research study (unpublished data). Specifically, the decision points examined and the subsequent findings are more likely to have greater external validity and higher fidelity to the unique needs of patients with MM.
Limitations of this study included the following; data were self- reported by patients and no verification of diagnosis, treatments, or -diagnostic tests was available. Therefore, recall bias may have affected results. In addition, because participants predominantly self-identified as Caucasian, non-Hispanic, and born in the United States, the survey findings may not represent the experiences of patients outside those patient groups. Lastly, no additional statistical analysis was performed in this study to control for selection bias and for potential confounding due to differences in demo- graphics between the MMRF-affiliated and nonaffiliated groups and their treatment decisions. This is an area of interest for future research.
Conclusion
Being involved with a trusted third-party organization, such as the MMRF, may empower patients to be better informed and more actively engaged in the management of their disease. As the concept of precision medicine advances in the MM research community, it remains critically important for patients and providers to supply key elements of data that will impact treatment decisions throughout the course of their disease. However, there are knowledge gaps that need to be addressed through patient-centered education and collaborative research initiatives, as these initiatives are intricately linked to developing customized treatment strategies and to accelerating genomic research discoveries. To achieve these bold objectives, third-party organizations appear to play a vital role in keeping patients well-informed from the very start of their journey from diagnosis through treatment.
Acknowledgements: The authors acknowledge the support of the Multiple Myeloma Research Foundation in funding this study and in assisting with participant recruitment. The authors sincerely thank Kenneth C. Ander- son, MD (program director, Jerome Lipper Multiple Myeloma Center and LeBow Institute for Myeloma Therapeutics at Dana-Farber Cancer Institute); Robert M. Rifkin, MD, FACP (associate chair, Hematology Research Committee, The US Oncology Network, Rocky Mountain Cancer Centers, Denver, Colorado); and A. Keith Stewart, MB, ChB (medical director, Center for Individualized Medicine at Mayo Clinic) for their critical review of the paper. The authors wish to acknowledge the editing assistance of Kerri Phillips, MBA, and Martine C. Maculaitis, PhD, on behalf of Kantar Health, with funding from the Multiple Myeloma Research Foundation.
Authors’ Disclosure of Potential Conflicts of Interest: Kathy Giusti has nothing to disclose in relation to this manuscript. Anne Q. Young has stock/ownership in Bluebird Bio. Melissa Winget is a consultant/ advisor for and has received research funding from the Multiple Myeloma Research Foundation. Kerri Lehrhaupt is a consultant/advisor for and has received research funding from the Multiple Myeloma Research Foundation.
Author Contributions: Conception and design: Kathy Giusti, Anne Q. Young, Kerri Lehrhaupt
Collection and assembly of data: Melissa Winget, Kerri Lehrhaupt
Data analysis and interpretation: Anne Q. Young, Melissa Winget, Kerri Lehrhaupt
Manuscript writing: All authors
Final approval of manuscript: All authors
Ethics Statement: The study was submitted and determined exempt by an independent Institutional Review Board (IRB) (Pearl IRB; Indianapolis, IN). Informed consent was obtained from all participants. Participants’ information was kept confidential, and all identifiers were removed prior to submission for publication.
Affiliations: Kathy Giusti and Anne Q. Young are with the Multiple Myeloma Research Foundation, Norwalk, Connecticut; Melissa Winget and Kerri Lehrhaupt are with Kantar Health, New York, New York.
Send correspondence to: Kerri Lehrhaupt, BSc, Vice President of Consulting Services; Kantar Health; 11 Madison Ave, 12th Floor; New York, NY 10010. Ph: (215) 997-6356; fax: (212) 647-7659; E-mail:
[email protected].
Disclaimers: The views expressed in this manuscript are solely those of the authors and do not reflect an official position of the authors’ institutions or those of the funding organization.







References
- Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385(9983):2197-2208. doi: 10.1016/S0140-6736(14)60493-1.
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046-1060. doi: 10.1056/NEJMra1011442.
- Keats JJ, Fonseca R, Chesi M, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12(2):131-144.
- Bianchi G, Munshi NC. Pathogenesis beyond the cancer clone(s) in multiple myeloma. Blood 2015;125(20):3049-3058. doi: 10.1182/ blood-2014-11-568881.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: myeloma. SEER website. http://seer.cancer.gov/statfacts/html/mulmy.html gov/statfacts/html/mulmy.html. Accessed April 7, 2016.
- American Cancer Society. Multiple myeloma. ACS website. http:// www.cancer.org/acs/groups/cid/documents/webcontent/003121-pdf.pdf. Published 2014. Updated January 19, 2016. Accessed April 7, 2016.
- Becker N. Epidemiology of multiple myeloma. In: Moehler T, Goldschmidt H, eds. Recent Results in Cancer Research. 183 edition. Berlin, Germany: Springer-Verlag; 2011:25-35.
- Dimopoulos M, Kyle R, Fermand JP, et al; International Myeloma Work- shop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705. doi.org/10.1182/blood-2010-10-299529.
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi: 10.1016/S1470- 2045(14)70442-5.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65(1):5-29. doi: 10.3322/caac.21254.
- Boland E, Eiser C, Ezaydi Y, Greenfield DM, Ahmedzai SH, Snowden JA. Living with advanced but stable multiple myeloma: a study of the symptom burden and cumulative effects of disease and intensive (hematopoietic stem cell transplant-based) treatment on health-related quality of life. J Pain Symptom Manage. 2013;46(5):671-680. doi: 10.1016/j.jpainsymman.2012.11.003.
- Mols F, Oerlemans S, Vos AH et al. Health-related quality of life and disease-specific complaints among multiple myeloma patients up to 10 yr after diagnosis: results from a population-based study using the PRO- FILES registry. Eur J Haematol. 2012;89(4):311-319. doi: 10.1111/j.1600- 0609.2012.01831.x.
- Goodwin JA, Coleman EA, Sullivan E, et al. Personal financial effects of multiple myeloma and its treatment. Cancer Nurs. 2013;36(4):301-308. doi: 10.1097/NCC.0b013e3182693522.
- Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008;111(5):2516-2520.
- Barlogie B, Mitchell A, van Rhee F, Epstein J, Morgan GJ, Crowley J. Curing myeloma at last: defining criteria and providing the evidence. Blood. 2014;124(20):3043-3051. doi: 10.1182/blood-2014-07-552059.
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793-795. doi: 10.1056/NEJMp1500523.
- National Institutes of Health. The Precision Medicine Initiative cohort program - building a research foundation for 21st century medicine. NIH website. http://acd.od.nih.gov/reports/DRAFT-PMI-WG-Re- port-9-11-2015-508.pdf.
- President Obama’s precision medicine initiative [press release]. Washington, DC: The White House Office of the Press Secretary; January 30, 2015. www.whitehouse.gov/the-press-office/2015/01/30/fact-sheet-president-obamas-precision-medicine-initiative. Accessed April 7, 2016.
- US National Institutes of Health. Relating clinical outcomes in multiple myeloma to personal assessment of genetic profile (CoM- Mpass). Clinical Trials website. https://clinicaltrials.gov/ct2/show/ NCT01454297?term=NCT01454297&rank=1. Accessed April 7 2016.
- Schulz PJ, Nakamoto K. Health literacy and patient empowerment in health communication: the importance of separating conjoined twins. Patient Educ Couns. 2013;90(1):4-11. doi: 10.1016/j. pec.2012.09.006.
- Oerlemans S, Husson O, Mols F, et al. Perceived information provision and satisfaction among lymphoma and multiple myeloma survivors--results from a Dutch population-based study. Ann Hematol. 2012;91(10):1587-1595. doi: 10.1007/s00277-012-1495-1.
- Multiple Myeloma Research Foundation. About MMRF. MMRF website. http://www.themmrf.org/about-mmrf/. Accessed April 7, 2016.
- Busch EL, Martin C, DeWalt DA, Sandler RS. Functional health literacy, chemotherapy decisions, and outcomes among a colorectal cancer cohort. Cancer Control. 2015;22(1):95-101.
- Greene J, Hibbard JH. Why does patient activation matter? an examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med. 2012;27(5):520-526. doi: 10.1007/s11606-011-1931-2.
- Thorne S, Hislop TG, Kim-Sing C, Oglov V, Oliffe JL, Stajduhar KI. Changing communication needs and preferences across the cancer care trajectory: insights from the patient perspective. Support Care Cancer. 2014;22(4):1009-1015. doi: 10.1007/s00520-013-2056-4.


