Introduction
Triple-negative breast cancer (TNBC) is a unique classification of breast malignancy characterized by its lack of expression of the estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor (HER2) receptors. This phenotype accounts for approximately 15% of all invasive breast cancers and carries a particularly poor prognosis due to its biologically aggressive nature and com- pounded by the lack of targeted therapy available for use.
There are several features of the triple-negative phenotype that have shifted our understanding TNBC as a unique entity within the breast cancer classification. Several epidemiological factors such as age, ethnicity, and body mass index (BMI) have been hypothesized to affect survival outcome in a TNBC population.1-8 Studies have demonstrated that the TNBC phenotype is a unique entity among breast cancer types showing higher rates of disease in younger women and women of African or Hispanic ancestry as well as women with higher BMI.
Two clinicopathologic variables which have been investigated among a greater breast population, however deserve more investigation specifically within the triple negative (TN) histology are lymphovascular invasion (LVI) and the use of preoperative MRI. LVI is an important component in the complex process of tumor metastasis but its prognostic value for various breast cancer types remains debatable.9-13 Current literature also suggests that imaging features of TNBC differ significantly from other breast cancer types,14 which may ultimately affect surgical intervention.
Understanding these variables in the larger context of management options may offer an opportunity to tailor a multimodality treatment course particularly for a disease which has no known specific therapeutic targets. In this study, we investigated the high-risk group of breast cancer with the triple-negative phenotype and sought to identify a potential panel of prognostic factors unique to this aggressive tumor type.
Methods
Study Population
We collected information through the University of Texas Southwest- ern tumor database on all consecutive patients diagnosed and treated with TNBC between March 1998 and September 2013. Patients with stage I to III invasive TNBC were eligible for inclusion.
Data on the patient’s age, race, social and medical history, type of surgery, chemotherapy, radiation therapy, clinical and pathological evaluation, staging, and tumor markers were available for all patients. A panel of ER, PR, and HER2 was used as a means of classifying breast cancer into the triple-negative molecular subtype. TNBC was defined as estrogen or progesterone immunohistochemical (IHC) staining <1% nuclei in 10 high-power fields and HER2 negativity was confirmed by (IHC) staining and fluorescence in situ hybridization (FISH) in cases that were equivocal. Lymphovascular invasion was assessed using hematoxylin and eosin (H&E) stain on full biopsy and pathology sections. Blood and lymphatic vessel invasion were not distinguished, and lymphovascular invasion was defined as positive if present on either biopsy or pathology.
Disease-free recurrence was calculated from last day of treatment to confirmation of recurrent disease in the ipsilateral breast, regional, or distant site. Recurrence in the ipsilateral treated breast and/or chest wall or ipsilateral nodal basin was considered locoregional recurrence (LR). Locoregional progression free survival (LPFS) was defined as those patients who either had locoregional recurrence or died. Any recurrence at a distant site was considered distant metastasis (DM). For patients who remained alive and recurrence-free, data were censored at the date of last follow-up. Overall survival (OS) was measured as the time from last day of treatment to death or last follow-up.
Statistical Analysis
Categorical variables were summarized as frequencies and percentages while continuous variables were reported as means and standard deviations. Kaplan-Meier survival curves for OS, DFS, and LPFS of patients for all prognostic factors were estimated and compared using log-rank tests. OS, DFS, and LPFS were censored after 5 years. All analyses were 2-sided and significance was set at a P value of .05. To assess if each prognostic factor was independently associated to OS, DFS, and LPFS after controlling for other clinical characteristics, all prognostic factors statistically significant at P < .05 in univariate analyses were included in a multivariate Cox proportional hazard regression model (Table 2 and Table 3). All analyses were done using IBM SPSS Statistics 22.0 software.
Results
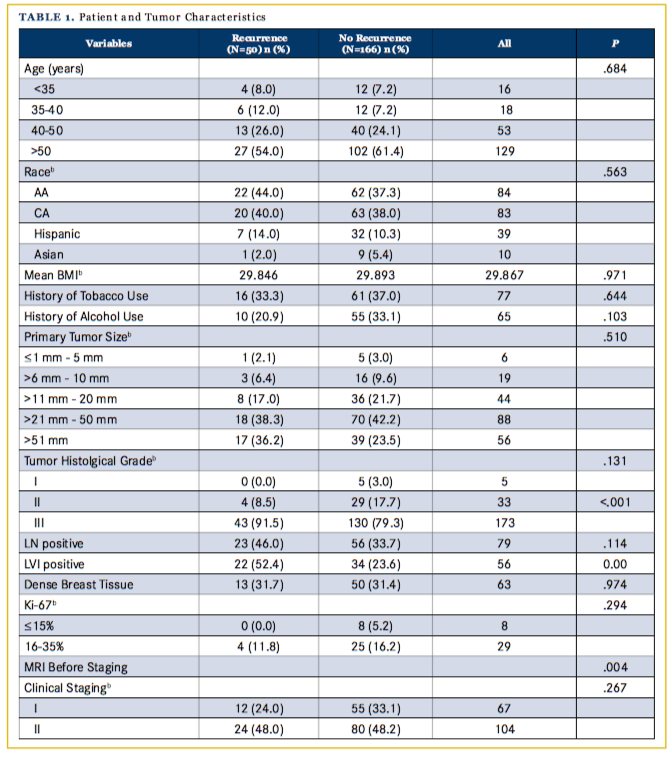
Of the 311 women included in the database, 277 satisfied the criteria for invasive TNBC as defined in this paper. Of these, 8 patients were both clinically stage 0 and were excluded from the analyses; 34 patients were both clinically and pathologically stage 4 and were also excluded. Three patients had unstageable disease (lymphadenopathy with no evidence of primary site), and 16 patients had an unknown clinical stage. Of the remaining 216 patients, 16 were ≤ 35 years old at the time of diagnosis and 200 were >35. The median age at diagnosis was 53 years. There were 84 African-American (AA), 83 Caucasian American (CA), 39 Hispanic, and 10 Asian women in the analyses sample. The median follow-up for all patients was 22.9 months. Differences in demographic, pathologic, and treatment-related characteristics between patients with and without recurrence are reported in Table 1. The overall and disease-free survival rates (DFS) over the 5-year study follow-up period for all patients were 82.8% and 77.2%, respectively.
Ethnicity Comparison:
The OS, DFS, and LPFS for women in different racial categories were examined using Kaplan-Meier survival curves and were compared using log-rank test. There was no statistically significant difference in OS (log-rank chi-square = 6.046, d.f = 3, P = .109), DFS (log-rank chi- square = 2.514, d.f.=3, P = .473) or LPFS (log-rank chi-square = 6.273, d.f = 3, P = .099) among the racial groups. Five-year OS rates for AA, CA, Hispanic, and Asian were 23.4, 64.8, 79.3, 100.0%, respectively, 5-year DFS rates 0.0, 58.6, 69.1, and 88.9%, and 5-year LPFS rates were 21.8, 59.8, 74.0, and 100.0%. In a direct comparison between AA versus CA women, AA women had significantly lower OS [23.4% vs. 64.8%, hazard ratio = 2.192, 95% confidence interval (CI) = 1.058-4.543, P = .04] rate over 5 years compared with CA women. Furthermore, AA women had a significantly longer time delay from diagnosis to treatment when compared with CA women (61 versus 43 days, respectively, P = .005).
Age Group Comparison
Women younger than 35 years were not significantly different from women older than 35 years in terms of OS (log-rank chi-square = 2.238, d.f. = 1, P = .135), DFS (log-rank chi-square = 0.025, d.f. = 1, P = .875), or LPFS (log-rank chi-square = 0.014, d.f. = 1, P = .906). 5-year OS for age <35 was 90.9% and 30.3% for >35, 5-year DFS was 55.4% and 46.8% respectively, and 5-year LPFS was 67.3% and 51.2%, respectively.
BMI Comparison
Obesity (BMI > 30) was not found to significantly influence OS (log-rank chi-square = 0.631, d.f. = 1, P = .427), DFS (log-rank chi-square = 0.040, d.f. = 1, P = .842), or LPFS (log-rank chi-square = 0.061, d.f = 1, P = .805) over the 5-year follow-up period.
Tobacco and Alcohol Use Comparison
Any history of tobacco use was not found to significantly influence OS (P = .969), DFS (P = .310), or LPFS (P = .371). Current tobacco use was also not found to significantly influence OS (P = .179), DFS (P = .486), or LPFS (P = .075). Neither any history of alcohol consumption nor current alcohol consumption was found to significantly influence OS (P = .465 and P = .514 for any history and current use, respectively), DFS (P = .136, P = .161), or LPFS (P = .365, P = .424).
Breast Density Comparison
Breast density was not found to significantly influence OS (log-rank chi-square = .481, d.f. = 1, P = .488), DFS (log-rank chi-square = .166, d.f. = 1, P = .683), or LPFS (log-rank chi-square = .117, d.f = 1, P = .732) over the 5-year follow-up period.
Lymphovascular Invasion Comparison
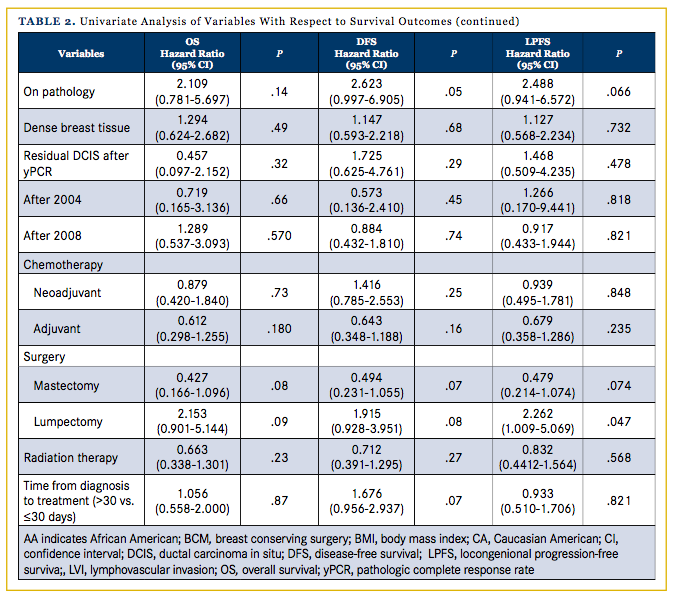
The presence of LVI stands as a significant predictor for OS (log-rank chi-square = 14.936, d.f. = 1, P < .001), DFS (log-rank chi-square = 15.566, d.f. = 1, P < .001), and LPFS (log-rank chi-square = 20.393, d.f. = 1, P < .001)
Preoperative MRI Comparison
A total 72 women (33%) underwent preoperative MRI. Patients who underwent a preoperative MRI were found to have improved DFS (log-rank chi-square = 6.907, d.f. = 1, P = .009) but not OS (log-rank chi-square = 2.558, d.f. = 1, P = .110) or LPFS (log-rank chi-square = 1.455, d.f. = 1, P = .228). Comparison of the preoperative MRI group and non-preoperative MRI group revealed that both groups were statistically similar regarding features such as race, stage, surgery type, and age. Women with dense breasts were more likely to receive a preoperative MRI. Of the 311 women included in the database, 277 satisfied the criteria for invasive TNBC as defined in this paper. Of these, 8 patients were both clinically stage 0 and were excluded from the analyses; 34 patients were both clinically and pathologically stage 4 and were also excluded. Three patients had unstageable disease (lymphadenopathy with no evidence of primary site) and 16 patients had an unknown clinical stage. Of the remaining 216 patients, 16 were younger than 35 years old at the time of diagnosis and 200 were older than 35 years. The median age at diagnosis was 53 years. There were 84 AA, 83 CA, 39 Hispanic, and 10 Asian women in the analyses sample. The median follow-up for all patients was 22.9 months. Differences in demographic, pathologic, and treatment-related characteristics between patients with and without recurrence are reported in Table 1. The overall and disease free survival rates over the 5-year study follow-up period for all patients were 82.8% and 77.2%, respectively.
Other Prognostic Factors
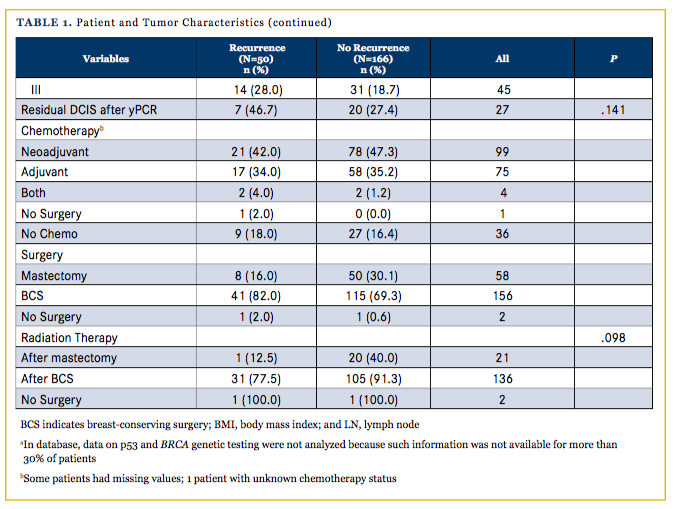
Advanced clinical stage was associated with significantly worsened outcomes in OS (P = .026) and DFS (P = .005) but not LPFS (P = .143). The proliferation marker Ki67 >35% as determined on pathology was associated with worse outcomes in OS (HR, 2.11; 95% CI, 0.78-5.70; P = .141), DFS (HR, 2.623; 95 % CI, 0.997-6.905; P = .051), and LPFS (HR, 2.488; 95% CI, 0.941-6.572; P = .066) over the 5-year follow-up period. A total of 157 women (73%) received radiation therapy either after lumpectomy or after mastectomy. Radiation treatment significantly decreased the rate of recurrence in our TNBC population (P <.001). Radiation therapy, however, was not shown to affect OS (HR, 0.66; 95% CI: 0.34-1.30; P =.232), DFS (HR, 0.71; 95% CI, 0.39-1.30; P = .266) or LPFS (HR, 0.832; CI, 0.44-1.56; P = .568). More days from diagnosis to first treatment trended with increased risk of death/ recurrence (DFS; HR, 1.676; 95% CI, 0.956-2.937). A total of 179 women (83%) received either neoadjuvant or adjuvant chemo- therapy. The presence of neoadjuvant or adjuvant chemotherapy was not found to significantly affect OS (P = .732 and P = .180 for neoadjuvant and adjuvant, respectively), DFS (P = .248, P = .159), or LPFS (P = .848, P = .235). The presence of residual ductal carcinoma in situ (DCIS) after pathologic complete response rate (yPCR) following neoadjuvant chemotherapy was also not found to significantly affect OS (P = .309), DFS (P = .286), or LPFS (P = .475).
Multivariate analysis
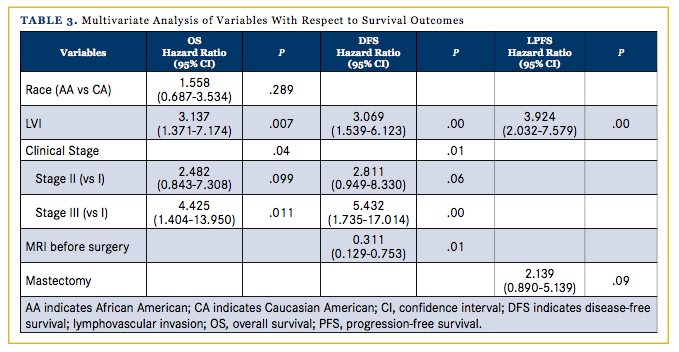
To assess if any of the above prognostic factors independently influenced OS, DFS, or LPFS over the 5-year follow-up period after controlling for other clinical and pathological characteristics, a multivariate Cox regression model was estimated. Race, LVI, preoperative MRI, clinical stage, and lumpectomy were included. LVI was the sole negative predictor of all 3 outcomes, OS (P = .002), DFS (P = .001), and LPFS (P <.001), over the 5-year follow-up period. Advanced clinical stage significantly predicted for worsened outcomes in OS (P = .043) and DFS (P = .012), and the presence of preoperative MRI significantly predicted for better DFS (P = .010).
Discussion
In our investigation, we determined advanced clinical stage, the presence of LVI and the use of preoperative MRI as the most significant predictors for outcome in a triple-negative population. Additionally, radiation was a significant predictor of recurrence in this early-stage population of TNBC women. Of note, women younger than 35 years of age did exhibit a non-significant decreased in 5-year OS compared with women older than 35; how- ever, this difference is likely a reflection of an imbalanced patient population between these 2 age groups.
When understood within the larger context of breast cancer, LVI has been shown to predict for an increased risk of local recurrence for women receiving both breast conservation therapy as well as mastectomy.16-20 LVI has also been associated with worse breast cancer-specific survival and distant metastasis-free survival.21 Unique to the triple-negative population in our study however, almost a quarter of women exhibited the presence of lymphovascular invasion. This number is disproportionately greater than the 15% reported elsewhere in the literature for invasive mammary ductal carcinomas of all types. Unique to this study was the correlation of LVI not only to higher local recurrence rates, but also to overall survival outcomes. In this population, LVI significantly predicted for OS, DFS, and LPFS.
Pages et al demonstrated in their evaluation of 959 colorectal specimens that cells lacking lymphovascular invasion was associated with T-cell infiltration, suggesting a beneficial role of the host’s immune system.22 Furthermore, they illustrated that tumors with a high level of T-cell infiltration were significantly associated with longer overall survival and disease-free survival.22 More work is needed to investigate whether a similar relationship exists between LVI and T-cell infiltration; however, previous studies have shown tumor infiltrating lymphocytes similarly carry a good prognostic significance for TNBC in 2 randomized clinical trials (ECOG 2197 and ECOG 1199)23 and has been shown to predict a pathologic complete response (pCR) to neoadjuvant chemotherapy.24 The exact balance between this lymphocytic infiltration, LI, and the potential targeting with immune specific therapy should be a continued area of exploration for TNBC.
The prognostic role of radiation therapy in an early TNBC population reflects recent findings by Abdulkarim et al. These authors illustrated in a recent Journal of Clinical Oncology study that the more conservative approach of partial mastectomy followed by radiation therapy resulted in improved locoregional control than mastectomy alone for early-stage breast cancer patients.25 In an associated commentary, Pignol et al posited an explanation for this finding and questioned whether radiation alters the molecular background of the breast tissue, as was seen with the anti-tumoral effect of wound fluids in the setting of intra-operative radiotherapy for accelerated partial breast irradiation.26 It is unclear what role radiation may play for triple-negative women, but recent studies have shown that post-mastectomy radiation can reduce locoregional recurrence and increase survival for T1-2 N1 ER negative women with LVI.27 Interestingly, the greatest proportion of women with LVI in the Abdulkarim publication was found within the group receiving breast conservation therapy, which included radiation. Our current investigation was not designed to detect the relationship between LVI and radiation therapy; however, this would be an important area of further investigation understand- ing the prognostic significance in our study.
Our study identifies a third important prognostic finding in this population, which was that of preoperative MRI. For the general breast population, MR mammography has been shown to be an important diagnostic tool for women with dense breasts,28 however, this has not generally been linked to improved outcomes.29-31 We found in this triple-negative population that women who underwent breast MRI prior to surgery had a significantly improved DFS. This is similar to the Bae et al findings that illustrated that the absence of preoperative MRI and the presence of dense breasts were associated with higher risk of local recurrence in a triple-negative population.32 Prior studies have shown that MRI carries the advantage in preoperative planning of showing full tumor extent, extensive intraductal component (EIC), and the multifocal or multicentric nature of the tumor.33,34 Furthermore, almost 20% of TNBCs are occult on initial mammography,35-37 lacking the characteristic features such as speculated margins with associated calcifications.35-37 The significance of MR for this patient population could provide more accurate and informative staging, as well as better target delineation in the setting of lumpectomy. Whether this shifts MRI to a more central component in the work up and surgical planning of TNBC warrants further study and consideration.
AA women in our evaluation had significantly lower OS rate over 5 years compared with CA women. Additionally, AA women had a significantly longer time delay from diagnosis to treatment compared with CA women (61 days vs 43 days, respectively). This highlights a particular disparity that needs further elucidation. In a 2013 JAMA review analyzing AA breast cancer patients, Silber et al illustrated the epidemic of delayed treatment and under-diagnosis among AA women. They showed that 12.6% of AA patients did not receive any treatment (vs 5.9% of white patients) and the average time from diagnosis to treatment was 29.2 days for AA patients (vs 22.5 days for white patients). These findings reflect the detrimental influence of disparity on health outcomes to which our study alludes and in the setting of TNBC could have significant implication.
Our study was limited by the retrospective nature of the investigation. In particular, there is limitation regarding our ability to report the indications for the decision making for preoperative MRI. Our analysis did capture that women with dense breasts were more likely to receive preoperative MRI; however, there is a high likelihood that the patients treated within the earlier portion of this 15-year period would be less likely to receive MRI due to a decreased availability and general practice. There is similar limitation with our ability to report the indications for the administration of chemotherapy or radiation given the retrospective nature of the study. In general, patients receiving partial mastectomy as well as mastectomy with positive lymph nodes, would have received postmastectomy radiation therapy. The majority of patients would have received chemotherapy unless the tumor was very small (ie, T1a) and/or a patient exhibited a poor performance status.
Conclusion
The presence of LVI stands as a significant predictor for OS and DFS, as well as locoregional control among a TN population. Additionally, the presence of preoperative MRI predicted for improved DFS among all groups. AA women experienced a significantly more delayed time from diagnosis to treatment com- pared with CA women. Future studies should explore the breast cancer management when faced with the unique characteristics of triple-negative disease, as well as the potential barriers to access and other discrepancies which could contribute to these findings.
Acknowledgments: We would like to acknowledge the staff, faculty, and patients at the University of Texas Southwestern for their help in conducting this research.
Author affiliations: All authors are from the University of Texas Southwestern Medical Center, Dallas, Texas.
Financial disclosure: None.
Author disclosures: Asal Rahimi, MD, MS, has provided consulting and advisory services (not related to this manuscript) to Genomic Health.
Presentation: The preliminary findings of this study were presented at the 2016 Miami Breast Conference.
Address correspondence to: Kimberly Thomas, MD, MSc, 5801 Forest Park Rd, Dallas, TX, 75235. Phone: (214) 645-2337; e-mail: [email protected].
References
- Sachdev JC, Ahmed S, Mirza MM, Farooq A, Kronish L, Jahanzeb M. Does race affect outcomes in triple negative breast cancer? Breast Cancer (Auckl). 2010;4:23-33.
- Lund MJ, Trivers KF, Porter PL, et al. Breast Cancer Res Treat. 2009;113(2):357-370. doi: 10.1007/s10549-008-9926-3.
- Bauer KR1, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor PR negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer. 2007;109(9):1721-1728.
- Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16 (suppl 1):1-11. doi: 10.1634/ theoncologist.2011-S1-01.
- Wei XQ, Li X, Xin XJ, Tong ZS, Zhang S. Clinical features and survival analysis of very young (age<35) breast cancer patients. Asian Pac J Cancer Prev. 2013;14(10):5949-5952.
- Lara-Medina F, Pérez-Sánchez V, Saavedra-Pérez D, et al. Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011;117(16):3658-3669. doi: 10.1002/cncr.25961.
- Turkoz FP, Solak M, Petekkaya I, et al. The prognostic impact of obesity on molecular subtypes of breast cancer in premenopausal women. J BUON. 2013;18(2):335-341.
- Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118(22):5463-5472. doi: 10.1002/cncr.27581.
- Mohammed RA, Martin SG, Gill MS, Green AR, Paish EC, Ellis IO. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequenc- es. Am J Surg Pathol. 2007;31(12):1825-1833.
- Rakha EA, Martin S, Lee AH, et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2012;118(15):3670-3680. doi: 10.1002/cncr.26711.
- Freedman GM, Li T, Polli LV, et al. Lymphatic space invasion is not an independent predictor of outcomes in early stage breast cancer treated by breast-conserving surgery and radiation. Breast J. 2012;18(5):415-419. doi: 10.1111/j.1524- 4741.2012.01271.x.
- Mohammed ZM, McMillan DC, Edwards J, et al. The relationship between lymphovascular invasion and angiogenesis, hormone receptors, cell proliferation and survival in patients with primary operable invasive ductal breast cancer. BMC Clin Pathol. 2013;13(1):31. doi: 10.1186/1472-6890-13-31.
- Ovcaricek T, Frkovic SG, Matos E, Mozina B, Borstnar S. Triple negative breast cancer — prognostic factors and survival. Radiol Oncol. 2011;45(1):46-52. doi: 10.2478/v10019-010-0054-4.
- Dogan BE, Turnbull LW: Imaging of triple-negative breast cancer. Ann Oncol. 2012;23(suppl 6):vi23-vi29
- Christoudias MK CA, Stull TS, Gracely EJ, Frazier TG, Barrio AV. Are the American Society for Radiation Oncology guidelines accurate predictors of recurrence in early stage breast cancer patients treated with balloon-based brachytherapy? Int J Surg Oncol. 2013;2013:829050. doi: 10.1155/2013/829050.
- Fisher ER, Anderson S, Tan-Chiu E, Fisher B, Eaton L, Wolmark N. Fifteen-year prognostic discriminants for invasive breast carcinoma: National Surgical Adjuvant Breast and Bowel Project Protocol-06. Cancer. 2001;91suppl 8:1679-1687.
- Fisher ER, Fisher B, Sass R, Wickerham L. Pathologic findings from the National Surgical Adjuvant Breast Project (Protocol No. 4). XI. Bilateral breast cancer. Cancer. 1984;54(12):3002-3011.
- Gajdos C, Tartter PI, Bleiweiss IJ. Lymphatic invasion, tumor size, and age are independent predictors of axillary lymph node metastases in women with T1 breast cancers. Ann Surg. 1999;230(5):692-696.
- Lauria R, Perrone F, Carlomagno C, et al. The prognostic value of lymphatic and blood vessel invasion in operable breast cancer. Cancer. 1995;76(10):1772-1778.
- Pinder SE, Ellis IO, Galea M, O’Rouke S, Blamey RW, Elston CW. Pathological prognostic factors in breast cancer. III. Vascular invasion: relationship with recurrence and survival in a large study with long-term follow-up. Histopathology. 1994;24(1):41-47.
- Rakha EA, Martin S, Lee AH, et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2012;118(15):3670-3680. doi: 10.1002/cncr.26711.
- Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654-2666.
- Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959-2966.
- Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105-113. doi: 10.1200/JCO.2009.23.7370.
- Abdulkarim BS, Cuartero J, Hanson J, Deschênes J, Lesniak D, Sabri S. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011;29(21):2852-2858. doi: 10.1200/JCO.2010.33.4714.
- Belletti B, Vaidya JS, D’Andrea S, et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res. 2008;14(5):1325-32. doi: 10.1158/1078-0432.CCR-07-4453
- Yang PS, Chen CM, Liu MC, et al. Radiotherapy can decrease locoregional recurrence and increase survival in mastectomy patients with T1 to T2 breast cancer and one to three positive nodes with negative estrogen receptor and positive lymphovascular invasion status. Int J Radiat Oncol Biol Phys. 2010;77(2):516-522. doi: 10.1016/j.ijrobp.2009.05.016.
- Sardanelli F, Giuseppetti GM, Panizza P, et al. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in fatty and dense breasts using the whole-breast pathologic examination as a gold standard. AJR Am J Roentgenol. 2004;183(4):1149-1157.
- Solin LJ, Orel SG, Hwang WT, Harris EE, Schnall MD. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women 1. with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol. 2008;26(3):386-391. doi: 10.1200/ JCO.2006.09.5448.
- Houssami N, Turner R, Macaskill P, et al. An individual person data meta-analysis of preoperative magnetic resonance imaging and breast cancer recurrence. J Clin Oncol. 2014;32(5):392- 401. doi: 10.1200/JCO.2013.52.7515.
- Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA Cancer J Clin. 2009;59(5):290-302. doi: 10.3322/caac.20028.
- Bae MS, Moon HG, Han W, et al. Early stage triple-negative breast cancer: imaging and clinical-pathologic factors associated with recurrence. Radiology. 2016;278:356-364. doi: 10.1148/radi- ol.2015150089.
- Oellinger H HS, Sander B, et al Gd-DTPA enhanced MRI of breast: the most sensitive method for detecting multicentric carcinomas in female breast? European Radiology 1993;3:223-226.
- Boetes C MR, Holland R, et al. Breast tumors: comparative accuracy of MR imaging relative to mammography and US for demonstrating extent. Radiology. 2016;278(2):356-364. doi: 10.1148/radiol.2015150089.
- Yang WT, Dryden M, Broglio K, et al. Mammographic features of triple receptor-negative primary breast cancers in young premenopausal women. Breast Cancer Res Treat. 2008;111(3):405-410.
- Wang Y, Ikeda DM, Narasimhan B, et al. Estrogen receptor-negative invasive breast cancer: imaging features of tumors with and without human epidermal growth factor receptor type 2 overexpression. Radiology. 2008;246(2):367-375. doi: 10.1148/ radiol.2462070169.
- Dogan BE, Gonzalez-Angulo AM, Gilcrease M, Dryden MJ, Yang WT. Multimodality imaging of triple receptor-negative tumors with mammography, ultrasound, and MRI. AJR Am J Roentgenol. 2010;194(4):1160-1166. doi: 10.2214/AJR.09.2355.