Introduction
Although there currently are 6 classes of drugs available for the treatment of multiple myeloma (MM; Table 1), the disease remains incurable in most cases, with patients eventually relapsing on each of these agents. The benchmark for drug approval based on 2 agents approved by the Food & Drug Administration (FDA) prior to 2015 (carfilzomib and pomalidomide) in the relapsed/refractory space was likely to be an overall response rate (ORR) of approximately 25% to 30% and progression-free survival (PFS) of approximately 4 months.1,2 That said, in less heavily pretreated patients with relapsed disease after 1 to 3 lines of therapy, given a high background rate of responses with already approved agents/regimens shown in Table 1, novel agents are also being increasingly approved by the FDA as a part of combination therapy based on PFS extension of 3 to 6 months achieved in randomized phase III studies. The goal of this review is to discuss the targets, mechanisms of action (MOA), safety, and efficacy of the novel agents in MM that are currently have recently met or have the best chance of meeting these benchmarks.
Targets of Immunomodulatory Drugs
Of note, there also have been developments in our understanding of the targets of immunomodulatory drugs (IMiDs). Until recently, it has been unclear how these oral medications achieve antimyeloma benefits without significant toxicity at the site of drug absorption in the gut or other off-target organ toxicities. Recently, cereblon, a member of the E3 ubiquitin ligase family, was identified as the likely mediator of teratogenicity of IMiDs. Cereblon also implicates IMiDs in the dysregulation of the ubiquitination process that targets proteins for proteasomaldegradation.3 Malignantplasmacells,byvirtueofbeing antibody/protein factories, have been demonstrated to be extremely vulnerable to such dysregulation by the proteasome inhibitor (PI) drug class.
Moreover, additional work suggests that the immunologic changes associated with IMiDs may be due to Aiolos and Ikaros, which are not only substrates of cereblon, but also transcription factors involved in interleukin 2–mediated T-cell activation.4 A better understanding of the MOA of these agents in MM will be essential to overcoming drug resistance and to developing the next generation of IMiDs and PIs. Indeed, CC-122, a so-called pleiotropic pathway modifier that targets cereblon and has antiproliferative, immunomodulatory, and antiangiogeneic activity, has now entered clinical trials.4
Monoclonal Antibodies and Other Immune-Mediated Therapies
There are several exciting developments in monoclonal antibody (mAb) use in MM (Table 2). To date, there has been no rituximab— that is, a mAb with single-agent activity—in MM. CD38 is a trans- membrane glycoprotein and ectoenzyme with high receptor density on MM cells.5 The CD38 antibodies daratumumab and isatuximab (SAR650984) are both humanized IgG1 mAbs with multiple MOA, including antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis, complement-dependent cytotoxicity, direct apoptosis induction, inhibition of CD38 enzymatic activity, T-cell clonal expansion, and functional response.6,7 Other than manageable infusional toxicities expected with mAbs, both agents are well tolerated. In a phase II study of 106 patients with a median of 5 lines of prior therapy, daratumumab monotherapy produced an ORR of 29%, with a median PFS of 3.7 months.8 Based on these results in a patient, daratumumab received accelerated FDA approval in November 2015 for patients with MM who have received more than 3 lines of therapy or are IMiD- and PI-refractory.
Combination therapy has also been explored with daratumumab. In the CASTOR study, the addition of daratumumab to bortezomib and dexamethasone resulted in an improvement in PFS (not reached [NR] vs 7.16 months; HR, 0.39; 95% CI, 0.28-0.53; P < .001); ORR (83 % vs 63%; P < .001); and complete response ([CR] 19 % vs 9%; P < .001).9 Similarly, in the POLLUX study, the addition of daratumumab to lenalidomide and dexamethasone led to an improvement in PFS (NR vs 18.4 mo; HR, 0.34; 95% CI, 0.23-0.48; P < .0001); ORR (93% vs 76%; P < .0001); CR (43% and 19%; P <.0001); including VGPR (76% vs 44%; P < .0001).10 On November 21, 2016, based on the above studies, the FDA approved daratumumab in combination withbothagents. Daratumumabpluspomalidomideisalsobeing investigated. In an ongoing, multicenter phase Ib trial in 77 patients, the ORR was 59%, including 58% in double-refractory patients. Generally speaking, the addition of daratumumab to the backbone agents in Table 1 has been with no added toxicity aside from daratumumab-related infusion reactions (Table 3).11
Isatuximab is a humanized mAb that binds selectively to human CD38receptor. IsatuximabbindstoadifferentepitopeonCD38 than daratumumab and may have more potent inhibition of its function. In a phase II trial in 97 patients with heavily pretreated MM, an RR of was oserve at a osae of m.12 In addition, it has been investigated in combination with lenalidomide, with an ORR of 64.5% and a PFS of 5.8 months (Table 3).13 An additional dose-escalation phase of this trial recently reported an ORR of 50% in 10 evaluable patients treated with isatuximab 20 mg/kg in combination with lenalidomide. Of note, 86% of patients treated in this dose-escalation study were lenalidomide-refractory.14Another mAb with activity in MM is elotuzumab, which is a humanized IgG1 mAb that targets cell surface 1 (CS1), a member of the SLAM family, which is uniformly and highly expressed in more than 95% of patients with primary MM, but not on stem cells or other normal tissues. The MOA appears to be ADCC-mediated by natural killer (NK) cells.15 Although elotuzumab has no significant single-agent activity, it has an excellent safety profile in combination with lenalidomide and dexamethasone. This was demonstrated in the phase III ELOQUENT-2 study, which randomized 646 patients with MM who had 1 to 3 lines of therapy prior to elotuzumab or placebo. All patients received a lenalidomide-and-dexamethasone backbone. The elotuzumab group had an ORR of 79% compared with the placebo group. Progression-free survival was 19.4 months in the elotuzumab group versus 14.9 months in the placebo group. Grades 3 and 4 toxicities were primarily hematologic, which were similar in the 2 groups aside from lymphocytopenia, which was significantly more common in the elotuzumab group. However, there was no significant increase in infections.16 Elotuzumab received FDA approval in November 2015 for more than 1 line of therapy given with lenalidomide-dexamethasone.
Elotuzumab has also been investigated in combination with bortezomibanddexamethasone.Onehundredandfifty-twopatients with 1 to 3 prior lines of therapy were randomized to elotuzumab, bortezomib, dexamethasone, or bortezomib and dexamethasone alone. Fifty-one percent in the treatment arm and 53% in the control arm had prior PI exposure. After a median number of 12 treatment cycles, PFS was 9.9 months in the elotuzumab group and 6.8 months in the control arm.17 Also of note, the CS1 target was now also being targeted by a mAb conjugated to the microtubule inhibitor monomethyl auristatin E (MMAE).18
Another mAb with an encouraging signal in MM is indatuximab ravtansine (formerly known as BT062), an anti-CD138 chimerized monoclonal IgG4 linked to the microtubule inhibitor maytansinoid (DM4). CD138 is primarily expressed on MM cells and also on epithelial cells, albeit to a lesser extent. Once the mAb binds to CD38, internalization and lysosomal processing of the linker releases the cytotoxic DM4 metabolites that result in apoptosis due to inhibition of tubulin polymerization.19 In a phase I/IIa trial of patients refractory to lenalidomide, the combination of indatuximab ravtansine plus lenalidomide produced a 78% ORR and 100% clinical benefit rate (CBR), including 73% in lenalidomide-refractory patients.20
As with other areas of oncology, there is increasing excitement about novel immune modulators in MM. Programmed cell death protein 1 (PD-1) is expressed on activated T-lymphocytes and engages its 2 cognate ligands (PD-L1 and PD-L2), causing inhibition of kinase pathways leading to attenuated T-lymphocyte activation. In healthy individuals, this pathway serves to downregulate unwanted or excessive immune responses.21 PD-L1 is also expressed on MM cells, allowingforimmuneevasion. Clinicaltrialsusingpembrolizumab, a highly selected anti-PD-1 mAb, is an attractive novel therapeutic for MM. Combination therapy of pembrolizumab with lenalidomide and dexamethasone has been explored in a phase I study of refractory patients produced an ORR of 76% in the 17 evaluable patients. Additionally, there was a 56% ORR in 9 lenalidomide-refractory patients.22 Pembrolizumab has also been explored in combination with pomalidomide and dexamethasone in a phase II trial of 24 patients, with an observed 50% ORR.23
Finally, as with other hematologic malignancies, there is great interest in chimeric antigen receptor (CAR) T-cell therapies. While a discussion of this modality is beyond the scope of this article, the targets being explored for this approach include CD19, CD38, CD40, CD44, CD47, ICAM1, NCAM1, CD74, CD81, CD86, CD200, IGF1R, CD307, CD317, SLAM7, PD-L1, CD138, and B-cell membrane antigen (BCMA).24 Of note, BCMA is also the target of a novel bispecific antibody manufactured by Janssen that will engage T cells.
Histone Deacetylase Inhibitors
Although MM cells are initially susceptible to PI therapy, the aggresome pathway is also responsible for the destruction of misfolded proteins. Dual-pathway inhibition has demonstrated preclinical synergy, perhaps due to the modulation of acetylation of histones and oncogenesis-related proteins (eg, p53, αtubulin, HIF-1α, hsp90).25,26 Two oral pan-histone deacetylase inhibitors (HDACs) have completed large phase III studies. The VANTAGE study comparing bortezomib and dexamethasone with either vorinostat or placebo showed only a disappointing 0.8-month improvement in PFS over the control arm.27
More recently, the PANORAMA 1 study with bortezomib (intravenous) and dexamethasone with either panobinostat or placebo showed a 3.9-month improvement in PFS and an improvement in CR rates from 15.7% to 27.6%. However, the rates of grade 3 or 4 diarrhea increased from 8% to 26%.28 Panobinostat was approved by the FDA on February 23, 2015, in combination with bortezomib and dexamethasone in patients who have received at least 2 prior regimens (including bortezomib and an IMiD). Importantly, PANORAMA 2 demonstrated that the addition of panobinostat to bortezomib in bortezomib-refractory patients resulted in a response rate (RR) of 34.5% and PFS of 5.4 months.29 In addition, when panobinostat is added to lenalidomide and dexamethasone, the ORR was 38% with a median PFS of 6.5 months, and more impressively, in the 22 lenalidomide-refractory patients, the ORR was 28% with a median PFS of 6.5 months.30
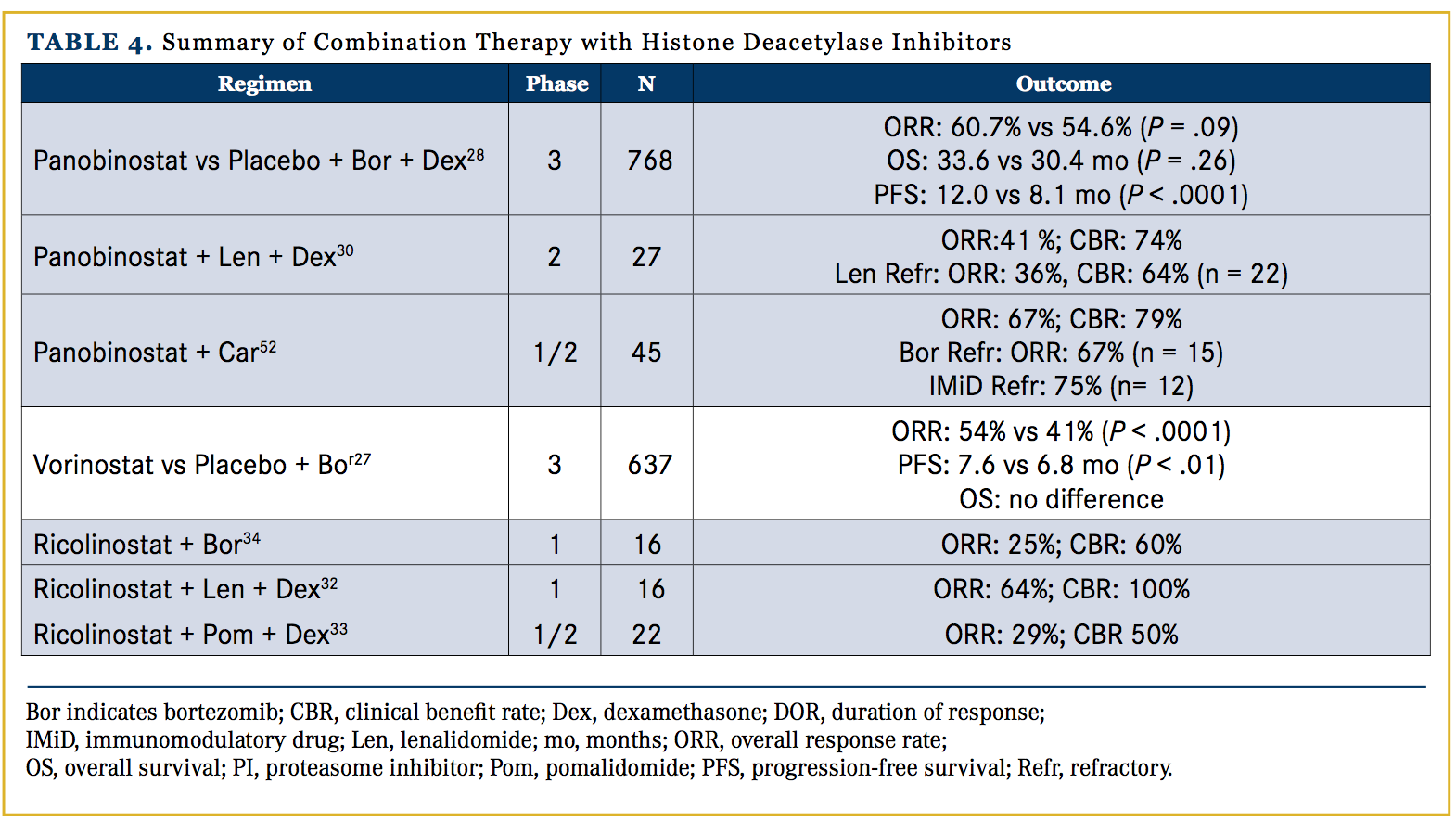
Ricolinostat (ACY-1215) is an oral selective HDAC6 inhibitor that has shown promising preclinical synergy with lenalidomide and pomalidomide.31 While tolerated as a monotherapy, it is currently being evaluated in the context of combination therapy. In combination with lenalidomide, it has demonstrated an ORR of 64%.32 In an ongoing phase I/II study of patients (median 4 lines of prior therapy), combination ricolinostat, pomalidomide, and dexamethasone demonstrated an ORR of 29%, including 3 VGPR.33 Additionally, ricolinostat combined with bortezomib and dexamethasone produced an ORR of 32% in 48 heavily pretreated patients (5 median lines of therapy).34 Currently available data on combination therapy with HDAC inhibitors are summarized in Table 4.
Non-mAb Novel Agents
There are additional non-mAb novel agents with activity in development (Table 5). Filanesib (ARRY-520) is a highly selective allosteric inhibitor of kinesin spindle protein (KSP), which is a microtubule motor protein. KSP inhibition prevents formation of a bipolar spindle, leading to apoptosis particularly in Mcl-1–dependent MM cells.35 The main toxicity is neutropenia, which is manageable with prophylactic granulocyte-colony stimulating factor (GCSF). As a single agent, the ORR is 16% and the median PFS is 3.7 months (n=32), which is comparable to the 15% and 3.4 months with the addition of dexamethasone to filanesib (n=55), albeit this was in a much more heavily pretreated, IMiD- and PI-refractory population.
Of particular interest, though, is the potential biomarker alpha 1-acid glycoprotein (AAG), an acute-phase protein used to monitor inflammatory conditions. AAG binds to filanesib, such that high AAG concentrations result in increased IC50 for filanesib in vitro. Lower AAG levels seem to correlate with clinical outcomes, as such patients had an ORR without dexamethasone of 24% and a median PFS of 5.1 months. Adverse events (AEs) were primarily hematologic.36 Although linking drug approval to a novel biomarker is not an easy path forward, to date, there are no biomarkers predictive of response in MM, and KSP inhibition is a novel mechanism of action in MM. Filanesib was granted orphan drug approval by the FDA in May 2014.
Filanesib has also been explored in combination therapy. A phase I study with bortezomib, filanesib, and dexamethasone in 55 patients (median 3 prior lines of therapy) produced an ORR of 20%, and was 29% for 14 PI-refractory patients receiving therapeutic doses of filanesib at >1.25 mg/m2.36 The responses ranged from 3.8 months to more than 24.6 months.37 In a phase II study, carfilzomib combined with filanesib or placebo (randomized in 2:1 fashion) demonstrated a 30% ORR in the combination arm versus 10% with carfilzomib alone.38
Another promising target is the phosphatidylinositide 3-kinase (PI3K)-AKT-mammalian target of rapamycin (mTOR) signaling pathway, which is activated in MM. In preclinical models, inhibition of this pathway leads to apoptosis.39 The oral, reversible, pan-AKT3 inhibitor afuresertib has been examined as a single agent in MM, demonstrating a favorable safety and pharmacokinetic profile, but on producing a RR of only 9%. The main toxicities were gastrointestinal (GI).40 Combination therapy with afuresertib, bortezomib, and dexamethasone may be more efficacious. This was demonstrated by a trial of 67 patients with relapsed MM where this combination produced an ORR of 61%.41 The pan-PIM kinase inhibitor LGH 447 has demonstrated an ORR of 10.5% in a phase I trial of heavily pretreated patients. While the single-agent responses for afuresertib and LGH 447 are modest, their use in combination therapy may be promising.42
Signal transduction inhibitors have been valuable therapeutic targets in other B-cell malignancies, but none have been approved for MM to date. Ibrutinib, an oral inhibitor of Bruton’s tyrosine kinase (BTK), is FDA-approved for treatment of chronic lymphocytic leukemia (CLL), mantle cell lymphoma, and Waldenström’s macroglobulinemia. In patients with MM, it produces a modest single-agent activity. In a phase II trial of ibrutinib, a cohort receiving ibrutinib 840 mg daily and dexamethasone experienced an ORR of 5% but a CBR of 25%.43 However, when combined with carfilzomib in a phase I trial of 40 patients, an ORR of 58% was achieved.44
Another related target is BCL-2, an antiapoptosis protein. Venetoclax is a potent, selective inhibitor of BCL-2 with efficacy in CLL and has been investigated as a single agent in MM. In a phase I study of 43 heavily pretreated patients (median 5 prior therapies), an ORR of 12% was observed. However, in patients with t(11;14) translocation, the ORR was 24%.45
Another novel target is cyclin-dependent kinases (CDKs), which regulate cell cycle progression. In MM, (Ig)H translocation can cause dysregulation of CDKs.46 Dinaciclib, a small molecular inhibitor of CDKs, has shown promise as a therapeutic agent in CLL and some solid tumors. It has been investigated as a single agent in a phase I/ II trial of 27 patients, producing an ORR of 11% and CBR of 19%.46 The most common AEs were hematologic (leukopenia and thrombocytopenia), GI-related, and fatigue.47
The nuclear protein exportin 1 (XP01) is a promising target in oncology. Preclinically, inhibition of XPO1 induced MM cytotoxicity and impaired osteoclastogenesis. Additionally, it may resensitize cells to bortezomib via blockade of NF-kB.48 Selinexor, a reversible XPO1 inhibitor, has been investigated in a phase I dose-escalation trial that included 29 patients with MM. A dosage of >65 mg produced 1 CR (3%), 6 partial responses (21%), and 6 minor responses (21%), giving an ORR of 24%. Selinexor was generally well tolerated, with primarily GI AEs reported.49 The addition of dexamethasone to selinexor was investigated in a phase I study of 28 patients with refractory MM with a median of 6 prior regimens. In 10 patients receiving selinexor 45 mg/ m2 and dexamethasone 20 mg, ORR was 60% (including 1 stringent CR and a CBR of 80%.50 This agent also has potential in heavily pretreated patients. An early press release of an ongoing phase IIb trial of selinexor reported an encouraging ORR of 20.8% in 48 quad-refractory patients and an ORR of 20% in 30 penta-refractory patients.51
Summary and Future Directions
For many decades, the treatment of MM was dependent upon only 2 classes of drugs: corticosteroids and conventional cytotoxics. In the past 10 years, the approval of PIs and IMiDs have made substantial contributions towards improving the outcomes of patients with MM, as evidenced by the fact that deletion of 13q and t(4;14) are no longer considered high risk. The approval of 4 new drugs in 2015, including 3 agents in novel classes (ie, HDAC and mAb), is truly remarkable for any cancer, let alone a relatively uncommon one such as MM. Although the single-agent activity (ie, ORR and PFS) of these agents is modest, rational combination therapies based on synergy and lack of overlapping toxicity is very encouraging. The true impact of these agents in groups of patients that remain at increased risk of death (eg, high molecular risk, frail/elderly, renal failure) is yet to be fully determined.
From a biologic perspective, improving upon this remarkable progress will require a better understanding of myelomagenesis, the mechanisms of resistance to current agents, and exploration of potential anti-MM targets. Similarly, future clinical trials will need to focus on biologically guided/risk-adapted therapy, continue to rapidly transition from the bench to bedside those agents with novel targets, and finally, use minimal residual disease to identify promising agents/ combinations to help accelerate drug approval. As outlined in this article, many promising targets hold the potential to build upon the already impressive progress made in the last decade.
Author affiliations: Douglas Tremblay, MD, and Ajai Chari, MD, are from the Mount Sinai School of Medicine, New York, NY.
Author disclosures: Dr. Tremblay has no disclosures. Dr. Chari has served as a consultant or is on a paid advisory board for Array Biopharma, Celgene, Millennium/Takeda, and Novartis, and has received grants from Array Biopharma, Celgene, Millennium/Takeda, Novartis, Onyx, GlaxoSmithKline, and Pharmacyclics.
Address correspondence to: Ajai Chari, MD, 1 Gustave Levy Place, Box 1185, New York, NY 10029; E-mail: [email protected].




References
- Siegel DS, Martin T, Wang M, et al. Aphase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817-2825. doi: 10.1182/ blood-2012-05-425934.
- San Miguel J, Weisel K, Moreau P, et al. Pomalidomideplus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055- 1066. doi: 10.1016/S1470-2045(13)70380-2.
- Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327(5971):1345-1350. doi: 10.1126/science.1177319.
- Hagner P, Wang M, Couto S, et al. CC-122 Has potent anti-lymphoma activity through destruction of the aiolos and ikaros transcription factors and induction of interferon response pathways. Blood. 2014;124(21):3035-3035.
- Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Path. 2004;121(4):482-488.
- Deckert J, Wetzel MC, Bartle LM, et al. SAR650984, a novel humanized CD38- targeting antibody, demonstrates potent anti-tumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res. 2014;20(17):4574-4583. doi: 10.1158/1078-0432.CCR-14-0695.
- Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune-regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016; 128(3):384-94. doi: 10.1182/blood-2015-12-687749.
- Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551- 60. doi: 10.1016/S0140-6736(15)01120-4.
- Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754-766. doi: 10.1056/NEJMoa1606038.
- Dimopoulos MA, Oriol A, Nahi H, et al. An open-label, randomised, Phase 3 study of daratumumab, lenalidomide, and dexamethasone (DRd) versus lenalidomide and dexamethasone (Rd) in relapsed or refractory multiple myeloma (RRMM): POLLUX. Paper presented at: EHA Annual Congress 2016.
- Chari A, Lonial S, Suvannasankha A, et al. Open-label, multi-center, phase 1b study of daratumumab in combination with pomalidomide and dexamethasone in patients with at least 2 lines of prior therapy and relapsed or relapsed and refractory multiple myeloma. Blood. 2015;126(23):508-508.
- Richter JR, Martin TG, Vij R, et al. Updated data from a phase II dose finding trial of single agent isatuximab (SAR650984, anti-CD38 mAb) in relapsed/refractory multiple myeloma (RRMM). 2016 ASCO Annual Meeting; 2016.
- Martin TG, Baz R, Benson DM, et al. A Phase Ib Dose Escalation Trial of SAR650984 (Anti-CD-38 mAb) in Combination with Lenalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma. Blood. 2014;124(21):83-83.
- Vij R, Lendvai N, Martin TG, et al. A phase Ib dose escalation trial of isatuximab (SAR650984, anti-CD38 mAb) plus lenalidomide and dexamethasone (Len/Dex) in relapsed/refractory multiple myeloma (RRMM): Interim results from two new dose cohorts. Paper presented at: ASCO Annual Meeting 2016.
- Hsi ED, Steinle R, Balasa B, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14(9):2775-2784. doi: 10.1158/1078-0432.CCR-07-4246.
- Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. New Engl J Med. 2015;373(7):621-631.
- Palumbo A, Offidani M, Pégourie B, et al. Elotuzumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma: 2-year follow-up. Blood. 2015;126(23):510.
- Gish K, Kim H, Powers R, et al. Preclinical evaluation of ABBV- 838, a first-in-call anti-CS1 antibody-drug conjugate for the treatment of multiple myeloma. Presented at: 2016 European Hematology Association Annual Congress; June 9-12, 2016; Copenhagen, Denmark. Abstract P644.
- Ikeda H, Hideshima T, Fulciniti M, et al. The monoclonal antibody nBT062 conjugated to cytotoxic maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clin Cancer Res. 2009;15(12):4028-4037. doi: 10.1158/1078-0432. CCR-08-2867. .
- Kelly KR, Chanan-Khan A, Heffner LT, et al. Indatuximab Ravtansine (BT062) in Combination with Lenalidomide and Low- Dose Dexamethasone in Patients with Relapsed and/or Refractory Multiple Myeloma: Clinical Activity in Patients Already Exposed to Lenalidomide and Bortezomib. Blood. 2014;124(21):4736-4736.
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. Oct 2 2000;192(7):1027-1034.
- Mateos M-V, Orlowski RZ, Siegel DSD, et al. Pembrolizumab in combination with lenalidomide and low-dose dexamethasone for relapsed/refractory multiple myeloma (RRMM): final efficacy and safety analysis. Presented at: 2016 American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. Abstract 8010..
- Badros AZ, Kocoglu MH, Ma N, et al. A phase II study of anti PD-1 antibody pembrolizumab, pomalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma (RRMM). Blood. 2015;126(23):506.
- Atanackovic D, Radhakrishnan SV, Bhardwaj N, Luetkens T. Chimeric antigen receptor (CAR) therapy for multiple myeloma. Br J Haematol. 2016;172(5):685-698. doi: 10.1111/bjh.13889. .
- Hideshima T, Richardson PG, Anderson KC. Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol Cancer Ther. 2011;10(11):2034-2042. doi: 10.1158/1535-7163.MCT-11-0433..
- Ocio EM, Vilanova D, Atadja P, et al. In vitro and in vivo ra- tionale for the triple combination of panobinostat (LBH589) and dexamethasone with either bortezomib or lenalidomide in multiple myeloma. Haematologica. 2010;95(5):794-803. doi: 10.3324/haematol.2009.015495..
- Dimopoulos M, Siegel DS, Lonial S, et al. Vorinostat or placebo in combination with bortezomib in patients with multiple myelo- ma (VANTAGE 088): a multicentre, randomised, double-blind study. Lancet Oncol. 2013;14(11):1129-1140. doi: 10.1016/S1470- 2045(13)70398-X.
- San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195-1206. doi: 10.1016/S1470- 2045(14)70440-1. .
- Richardson PG, Schlossman RL, Alsina M, et al. PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood. 2013;122(14):2331-2337. doi: 10.1182/blood-2013-01-481325..
- Chari A, Cho HJ, Leng S, et al. A phase II study of panobi- nostat with lenalidomide and weekly dexamethasone in myeloma. Blood. 2015;126(23):4226.
- Jones SS. ACY-1215, a First-In-Class Selective Inhibitor Of HDAC6, Demonstrates Significant Synergy With Immunomodulatory Drugs (IMiDs) In Preclinical Models Of Multiple Myeloma (MM). Blood. 2013;122(21):1952-1952.
- Yee AJ, Bensinger W, Voorhees PM, et al. Ricolinostat (ACY- 1215), the first selective HDAC6 inhibitor, in combination with lenalidomide and dexamethasone in patients with relapsed and relapsed-and-refractory multiple myeloma: phase 1b results (ACE- MM-101 Study). Blood. 2015;126(23):3055. doi: 10.1016/S1470- 2045(16)30375-8. .
- Raje NS, Bensinger W, Cole CE, et al. Ricolinostat (ACY-1215), the first selective HDAC6 inhibitor, combines safely with poma- lidomide and dexamethasone and shows promosing early results in relapsed-and-refractory myeloma (ACE-MM-102 Study). Blood. 2015;126(23):4228.
- Vogl DT, Hari PN, Jagannath S, et al. ACY-1215, a Selective Histone Deacetylase (HDAC) 6 Inhibitor: Interim Results Of Combination Therapy With Bortezomib In Patients With Multiple Myeloma (MM). Blood. 2013;122(21):759-759.
- Ocio EM, Richardson PG, Rajkumar SV, et al. New drugs and novel mechanisms of action in multiple myeloma in 2013: a report from the International Myeloma Working Group (IMWG). Leukemia. 2014;28(3):525-542. doi: 10.1038/leu.2013.350..
- Lonial S, Shah JJ, Zonder J, et al. Prolonged survival and improved response rates with ARRY-520 (filanesib) in relapsed/ refractor mtipe meoma (RRMM) patients with ow ‒ aci glycoprotein (AAG) levels: results from a phase 2 study. Blood. 2013;122(21):285.
- Chari A, Htut M, Zonder JA, et al. A phase 1 dose-escala- tion study of filanesib plus bortezomib and dexamethasone in patients with recurrent/refractory multiple myeloma. Cancer. 2016;122(21):3327-3335. doi: 10.1002/cncr.30174. .
- Zonder JA, Usmani S, Scott EC, et al. Phase 2 study of carfilzomib (CFZ) with or without filanesib (FIL) in patients with advanced multiple myeloma (MM). Blood. 2015;126(23):728.
- McMillin DW, Ooi M, Delmore J, et al. Antimyeloma activity of the orally bioavailable dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Cancer Res. 2009;69(14):5835-5842.
- Spencer A, Yoon S-S, Harrison SJ, et al. The novel AKT inhibitor afuresertib shows favorable safety, pharmacokinetics, and clinical activity in multiple myeloma. Blood. 2014;124(14):2190-2195. doi: 10.1182/blood-2014-03-559963. .
- Spencer A, Sutherland HJ, O’Dwyer ME, et al. Novel AKT inhibitor afuresertib in combination with bortezomib and dexamethasone demonstrates favorable safety profile and significant clinical activity in patients with relapsed/refractory multiple myeloma. Blood. 2013;122(21):283.
- Raab MS, Ocio EM, Thomas SK, et al. Phase 1 study update of the novel pan-Pim kinase inhibitor LGH447 in patients with relapsed/refractory multiple myeloma. Blood. 2014;124(21):301.
- Vij R, Huff CA, Bensinger WI, et al. Ibrutinib, single agent or in combination with dexamethasone, in patients with relapsed or relapsed/refractory multiple myeloma (MM): preliminary phase 2 results. Blood. 2014;124(21):31.
- Chari A, Chhabra S, Usmani S, et al. Combination treatment of the Bruton’s tyrosine kinase inhibitor ibrutinib and carfilzomib in patients with relapsed or relapsed and refractory multiple myeloma: initial results from a multicenter phase 1/2b study. Blood. 2015;126(23):377.
- Kumar S, Vij R, Kaufman JL, et al. Phase I venetoclax monotherapy for relapsed/refractory multiple myeloma. Presented at: 2016 American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. Abstract 8032.
- Bergsagel PL, Kuehl WM, Zhan F, et al. Cyclin D dysregulation: an early and unifying pathogenic event in multiple myeloma. Blood. 2005;106(1):296-303.
- Kumar SK, LaPlant B, Chng WJ, et al. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood. 2015;125(3):443- 448.
- Tai YT, Landesman Y, Acharya C, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2014;28(1):155-165.
- Chen C, Garzon R, Gutierrez M, et al. Safety, efficacy, and determination of the recommended phase 2 dose for the oral selective inhibitor of nuclear export (SINE) selinexor (KPT-330). Blood. 2015;126(23):258.
- Chen CI, Gutierrez M, Siegel DS, et al. Selinexor demonstrates marked synergy with dexamethasone (Sel-Dex) in preclinical models and in patients with heavily pretreated refractory multiple myeloma (MM). Blood. 2014;124(21):4773.
- Karyopharm reports positive top-line phase 2b STORM results and reviews the planned development path for selinexor in multiple myeloma [press release]. September 6, 2016. http:// investors.karyopharm.com/releasedetail.cfm?releaseid=987776. Accessed November 28, 2016.
- Berdeja JG, Hart LL, Mace JR, et al. Phase I/II study of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma. Haematologica. 2015;100(5):670-676.
- Chari A, Htut M, Zonder J, et al. A phase 1 study Of ARRY-520 with bortezomib (BTZ) and dexamethasone (dex) in relapsed or refractory multiple myeloma (RRMM). Blood. 2013;122(21):1938.
- Jakubowiak A, Jasielec J, Rosenbaum CA, et al. Phase 1 MMRC trial of selinexor, carfilzomib (CFZ), and dexamethasone (DEX) in relapsed and relapsed/refractory multiple myeloma (RRMM). Blood. 2015;126(23):4223.


