Hodgkin lymphoma (HL) has a well characterized bimodal distribution, and approximately 20% of patients diagnosed with HL are greater than 60 years old. Most patients diagnosed with Hodgkin lymphoma are cured with standard chemotherapy regimens with or without involved field radiation therapy. While older patients represent a relatively small number of new diagnoses each year, they represent a disproportionately large percentage of deaths. According to the Surveillance, Epidemiology and End Results (SEER) database, 51% of patients who die from Hodgkin lymphoma are over the age of 65.1 Historically, the outcomes of older patients with HL have been poor with an overall survival (OS) of 30% to 50% at 5 years.2-5 Improved survival of older patients with HL has been seen with 5-year OS of 58.8% in the 2000–2004 period compared with 34.8% in the 1980–1984 period.6 Older patients have been underrepresented on clinical trials, which is highlighted by a retrospective analysis by the German Hodgkin’s Study Group (GHSG) revealing that patients older than 60 years of age made up only 8.8% of the patients in the HD5-HD9 studies.7 In the next sections, we will outline the existing data on the outcomes and treatment approaches in older patients with HL highlighting this unmet need. We will also review recent data regarding the use of brentuximab vedotin (BV) and speculate on future approaches in this population.
Outcomes and Toxicity of Treatment in Older Patients
Multiple recent retrospective series have described the outcomes of older patients with HL. A multicenter cohort study by Evens and colleagues examined survival and toxicity outcomes of 95 patients with a median age of 67 years at diagnosis. Patients received a variety of treatments including various chemotherapy combinations, the most common of which were doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD), as well as, radiation, hospice, and watchful waiting. In this study, the 2- and 5-year OS were 73% and 58%, respectively. Being over 70 years of age and loss of activities of daily living (ADLs) were characteristics associated with inferior outcomes.8 A retrospective, multicenter analysis of 147 patients, all treated with ABVD, demonstrates similar survival with a 5-year OS of 67% with similar rates of toxicity.9
There have been secondary analyses of the older patients included on several large cooperative group trials. The GHSG compiled early stage patients with HL from HD10 and HD11 trials. There were statistically significant differences in acute toxicity when the older group was compared to the patients younger than 60 years old. Twenty-eight percent of older patients died during follow-up, compared to 4% of younger patients. Five percent of older patients experienced treatment-related mortality, compared to <1% in the younger group.10 The outcomes of advanced-stage patients older than 60 years from E2496 have been reported and compared to the outcomes of younger patients. Subjects were randomized between ABVD and Stanford V (doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide and prednisone). Five-year OS was 58% in older patients versus 90% in those younger than 60 years of age, which reached statistical significance. The difference in survival was felt to be due, in part, to competing risks for death and toxicity as there was no difference in the rate of death due to progressive disease.11
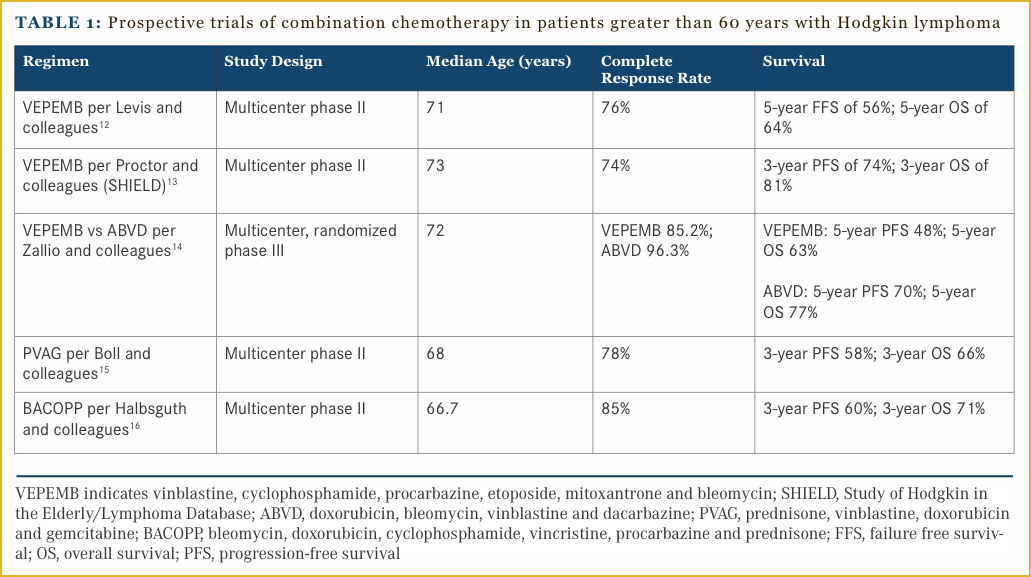
The prospective trials that have been conducted specifically in an older population, are limited by their small size and lack of randomization. A regimen comprised of vinblastine, cyclophosphamide, procarbazine, etoposide, mitoxantrone and bleomycin (VEPEMB) has been the subject of several single-arm trials and one randomized study. A multicenter Italian study enrolled 105 patients with a median age of 71 years who were treated with (VEPEMB) followed by involved field radiation. The outcomes in early-stage patients were excellent with a CR rate of 98% versus 58% in advanced-stage patients. The survival outcomes in advanced-stage patients were also inferior with a 79% 5-year failure free survival in early stage and only 34% in advanced stage.12 The Study of Hodgkin in the Elderly/Lymphoma Database (SHIELD) conducted in the United Kingdom enrolled patients onto a prospective phase II study of VEPEMB chemotherapy if they were deemed non-frail based on a comorbidity assessment. Patients who were considered frail were treated at the individual investigator’s discretion, and enrolled onto the registration portion of the study. The majority of the patients enrolled on the registry portion received ABVD as investigator’s choice therapy. In the early-stage patients 3-year OS and PFS were 81% and 74%, respectively. The 3-year OS and PFS in advanced-stage patients were 66% and 58%.13 This regimen has been compared to ABVD in a randomized trial that was recently reported. This study randomized 54 patients between the ages of 65 and 80 years to treatment with VEPEMB or ABVD. Importantly, these patients underwent a comprehensive geriatric assessment prior to enrollment and were considered non-frail. The primary endpoint of this study was PFS. There was a trend toward inferiority in the VEPEMB group in terms of 5-year PFS (48% vs 70%). Toxicity was low in each arm with only 2 pulmonary events in each arm and one toxic death in each arm, which may have been due to patient selection as these patients were all non-frail.14
The GHSG has conducted several prospective studies aimed at older patients with HL. A combination of prednisone, vinblastine, doxorubicin and gemcitabine (PVAG) has been studied by the GHSG in early unfavorable and advanced-stage HL. Patients between 60 and 75 years of age received 6-8 cycles of PVAG, followed by radiotherapy if they were not in CR. Seventy-eight percent of patients achieved a CR and the 3-year OS and PFS were 66% and 58%, respectively. Seventy-five percent of patients had grade 3-4 toxicities, but there was only one reported death.15 The GHSG conducted a phase II study of bleomycin, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone (BACOPP) in patients aged between 60 and 75 years. Eighty-five percent of patients achieved a CR. The 3-year PFS and OS were 60% and 71%, respectively.16 The prospective studies conducted in older patients are summarized in Table 1.
Bleomycin Pulmonary Toxicity
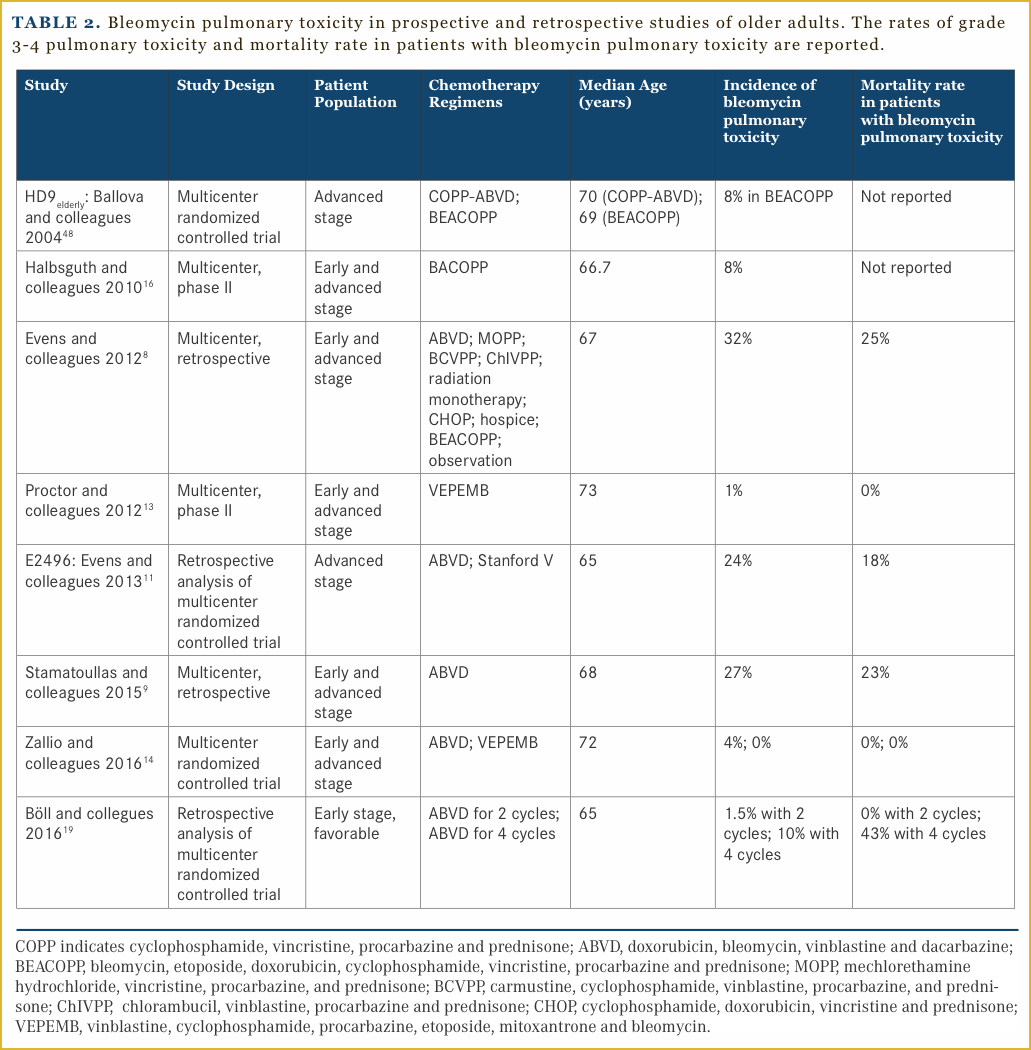
Bleomycin pulmonary toxicity is a devastating complication of therapy in older patients. It is characterized by the development of a nonproductive cough and exertional dyspnea, which can become progressive with lung scarring and reductions in the diffusion capacity. Risk factors for the development of bleomycin pulmonary toxicity include advanced age, smoking, renal impairment, and possibly granulocyte-colony stimulating factor administration.17,18 It is of particular concern in HL patients as the curative regimens that are considered standard of care, incorporate bleomycin. In various retrospective series, which included primarily patients who received ABVD, nearly one-third of older patients developed bleomycin pulmonary toxicity, which carries a substantial risk of death.8,9,11 In a single institution retrospective series in the United States, the median 5-year OS was 90% in those who did not develop bleomycin pulmonary toxicity versus 63% in those who did. This study included patients of all ages with a median age of 32 years. ABVD as initial therapy, increased age defined as greater than 40 years, and use of granulocyte colony-stimulating factor were associated with bleomycin pulmonary toxicity.17 The number of cycles of combination chemotherapy containing bleomycin that older patients received do seem to have an impact on the development of bleomycin pulmonary toxicity, as well as overall toxicity. Data from 287 older patients with early favorable HL enrolled on HD10 and HD13 were analyzed, and revealed that 2 cycles of ABVD compared to 2 cycles of AVD were equally tolerable in older patients. More toxicity was seen, including fatal bleomycin pulmonary toxicity in patients who received 4 cycles of ABVD.19 Table 2 summarizes data from prospective and retrospective studies involving older patients. These data support the study of regimens that limit bleomycin in older patients as well as the need to better risk stratify patients.
Brentuximab Vedotin as Initial Treatment
Because intolerance to standard chemotherapy contributes to the inferior outcomes in older patients, strategies such as dose escalation are unlikely to benefit this population. Highly effective, well tolerated, targeted agents are needed in this population. Targeting CD30 and checkpoint inhibition are two highly efficacious strategies in HL. BV is an anti-CD30 antibody-drug conjugate that has proven to be highly effective in relapsed CD30 positive malignancies including HL. BV binds to CD30 on the cell surface, is internalized and traffics to the lysosome. In the lysosome a peptide linker is cleaved, which releases a tubular toxin monomethyl auristatin E from the anti-CD30 monoclonal antibody. The pivotal trial of BV in HL relapsed after autologous stem cell transplant had an objective response rate (ORR) of 75% with 34% of subjects achieving a CR. While the median PFS was modest at 5.6 months, in those patients who achieved a CR, the duration of response was 20.5 months. The agent was safely given to this population with the most common, grade 3 events being peripheral sensory neuropathy and neutropenia at 8% and 14%, respectively.20 This led to accelerated approval of BV by the FDA. Based on the available safety and efficacy data in the relapsed setting, clinical trials incorporating BV into initial therapy have been designed.
A multi-cohort phase II study of BV, as a single agent and in combination, has been conducted as first-line therapy in older patients with HL. As a single agent, BV demonstrated an ORR of 92% with 73% of patients achieving a CR. The median age was over 70 years, and patients received a median of 8 cycles of therapy. Despite the excellent response rates, the responses were not durable with a median DOR of 9.1 months and median PFS of 10.1 months. While single-agent BV was well tolerated overall as initial therapy, nearly 30% of patients treated with single-agent BV developed grade 3 sensory peripheral neuropathy. Importantly there were no grade 3 pulmonary events in the monotherapy cohort and no episodes of febrile neutropenia.21 This study also included arms with BV in combination with dacarbazine and bendamustine. The ORR for patients treated with BV and dacarbazine was 100% with 62% achieving a CR and median PFS was not reached. Similarly, bendamustine and BV had an excellent ORR of 100%, but follow up is short. The combination of BV and dacarbazine had a better safety profile when compared to BV and bendamustine. In the arm with dacarbazine, patients received a median of 11.5 cycles and had a rate of grade 3 or greater treatment-related adverse events of 36%. Only 9% of patients experienced a severe adverse event. Forty-five percent of patients in the bendamustine arm experienced grade 3 or greater adverse events and nearly half of the patients experienced severe adverse events.22
There is no consensus regarding the management of older patients with HL. Salvage chemotherapy followed by consolidation of a response with an autologous stem cell transplant (ASCT) is the standard treatment in younger relapsed and refractory patients.23,24 Older patients with relapsed HL have an extremely poor prognosis, many of whom are not eligible for salvage chemotherapy and transplant due to age, frailty, and comorbidities. There has previously been no standard-of-care treatment in this situation. The German Hodgkin study group analyzed patients enrolled on their trials from 1993-2007, which comprised approximately 100 patients. The treatment strategies varied from palliative care to salvage regimens and conventional multi agent chemotherapy with radiation. Univariate analysis identified stage, early relapse, and anemia as predictive risk factors.25
Novel agents have presented new options for older patients with relapsed HL. BV is safe and effective in older patients. A retrospective analysis of patients with relapsed and refractory CD30-positive lymphomas identified 16 patients with HL. Fifty-six percent of patients with HL achieved an objective response with 38% achieving a CR. The median PFS was 9 months and duration of response was not reached.26 The preliminary results of clinical trials with the programmed cell death 1 (PD-1) inhibitors nivolumab and pembrolizumab are highly encouraging in relapsed HL, and checkpoint inhibition is recognized as a potentially highly active strategy in HL. There are few data on the use of checkpoint inhibition in older patients with relapsed and refractory HL, as two recent high profile studies with pembrolizumab and nivolumab had median ages of 28 and 35, respectively.27,28 There is now experience with these agents in a wide variety of cancer types including melanoma, lung carcinoma, renal cell carcinoma, and Merkel cell carcinoma among others. Patients over the age of 60, are well represented in many of these trials with median ages ranging from 61 to 68 years. These agents have been well tolerated in older patients with rates of grade 3 and greater toxicities ranging from 10% to 15%. Common adverse events include pneumonitis, diarrhea, fatigue and hypothyroidism.29-35
Predicting Toxicity
Older adults are heterogeneous with regards to their overall health status, and geriatric patents are vulnerable to a unique set of medical and social issues, such as cognitive impairment and falls, as compared to their younger counterparts. These vulnerabilities could impact decision making for cancer therapy,36 risks associated with treatment,37,38 and overall outcomes.39
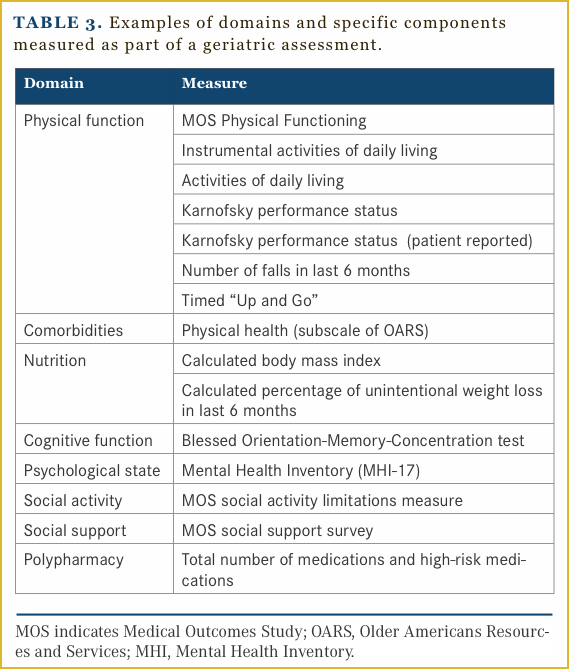
A geriatric assessment (GA) can help to provide a thorough understanding of an older adult’s health status and identify those individuals at increased risk for chemotherapy toxicity.37,38 GA has multiple components and typically includes evaluation of an older adult’s physical function, cognition function, comorbidities, psychological status, nutritional status, social support and medication review. A cancer-specific GA has been developed40 and is feasible to incorporate into oncology research and clinical practice.41,42 Table 3 provides a summary of components tested as part of a GA.
In hematologic malignancies, specifically, elements of the GA have been shown to add prognostic value.43,44 Clinical trials in older adults with Hodgkin lymphoma are now incorporating GA elements, as well. Forero-Tores and colleagues included GA variables in a phase II study of BV monotherapy in older adults with Hodgkin lymphoma.21 In this study, GA identified impairments in a significant proportion of the study population (81% with impaired physical function, 52% with significant comorbidity, 33% with malnutrition). An Italian study comparing a reduced-intensity regimen with standard therapy also incorporated GA elements, including physical function and geriatric syndromes, into eligibility criteria for the trial.45 Investigators observed low rates of toxicity and treatment-related mortality for both regimens in this study and attributed these low rates of adverse events to the stringent selection of subjects due to incorporation of GA elements.
Summary and Future Directions
Older patients with HL have a poor tolerance to standard combination chemotherapy. Treatment-related morbidity and mortality, particularly, bleomycin pulmonary toxicity, are serious concerns. Approaches aimed at identifying those at highest risk of serious toxicities, as well as more efficacious, better tolerated therapies are needed in this age group.
Significant strides are unlikely to be made in older patients with increased dose intensity with regimens such as escalated BEACOPP, and thus novel combinations need to be studied. Targeted therapies such as BV and checkpoint inhibition have shown unprecedented activity in patients with relapsed HL.20,27,28 Studies incorporating BV into first-line therapy for older patients are underway and there is substantial enthusiasm for studying combinations of targeted therapies in this age group.
Incorporation of GA into clinical trials for older adults with Hodgkin lymphoma may assist in clinical decision making, as practitioners will have a better understanding of the overall health status of elderly patients enrolled on specific clinical trials and the applicability of trial results to patient seen in routine clinical practice. GA may also aid in developing supportive care interventions to help older adults receiving cancer therapy, complete treatment, and thus derive optimal benefit.46,47
Improved treatment selection based on GA as well as the availability of highly active, targeted agents have the potential to dramatically alter the outcomes for older patients with HL. Studies designed to confirm the ability of the GA to identify patients who are at high risk for toxicity with standard combination chemotherapy are needed to avoid delivering therapy, indiscriminately, to patients who are unlikely to benefit from it. At the same time, studies exploring the use of novel agents alone or in combination with chemotherapy, are needed to improve the outcome in this high-risk population. Ongoing trials including a multicenter phase II study of BV and nivolumab in older patients NCT02758717 will help define future standards of care for this as yet unmet need.
Affiliations: Patrick M. Reagan, MD, Allison M. Magnuson, DO, and Jonathan W. Friedberg, MD, MMSc, are with the James P. Wilmot Cancer Center, Rochester, NY.
Address for correspondence: Patrick M. Reagan, MD, James P. Wilmot Cancer Center, 601 Elmwood Ave., Rochester, NY 14642. Phone: (585) 275-8762. E-mail: [email protected].
References
2. Mir R, Anderson J, Strauchen J, et al. Hodgkin disease in patients 60 years of age or older. Histologic and clinical features of advanced-stage disease. The Cancer and Leukemia Group B. Cancer. 1993;71(5):1857-1866.
3. Stark GL, Wood KM, Jack F, et al. Hodgkin’s disease in the elderly: a population-based study. Br J Haematol. 2002;119(2):432-440.
4. Weekes CD, Vose JM, Lynch JC, et al. Hodgkin’s disease in the elderly: improved treatment outcome with a doxorubicin-containing regimen. J Clin Oncol. 2002;20(4):1087-1093.
5. Landgren O, Algernon C, Axdorph U, et al. Hodgkin’s lymphoma in the elderly with special reference to type and intensity of chemotherapy in relation to prognosis. Haematologica. 2003;88(4):438-444.
6. Brenner H, Gondos A, Pulte D. Ongoing improvement in long-term survival of patients with Hodgkin disease at all ages and recent catch-up of older patients. Blood. 2008;111(6):2977-2983.
7. Engert A, Ballova V, Haverkamp H, et al. Hodgkin’s lymphoma in elderly patients: a comprehensive retrospective analysis from the German Hodgkin’s Study Group. J Clin Oncol. 2005;23(22):5052-5060.
8. Evens AM, Helenowski I, Ramsdale E, et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood. 2012;119(3):692-695. doi: 10.1182/blood-2011-09-378414.
9. Stamatoullas A, Brice P, Bouabdallah R, et al. Outcome of patients older than 60 years with classical Hodgkin lymphoma treated with front line ABVD chemotherapy: frequent pulmonary events suggest limiting the use of bleomycin in the elderly. Br J Haematol. 2015;170(2):179-184.
10. Böll B, Gorgen H, Fuchs M, et al. ABVD in older patients with early-stage Hodgkin lymphoma treated within the German Hodgkin Study Group HD10 and HD11 trials. J Clin Oncol. 2013;31(12):1522-1529. doi: 10.1200/JCO.2012.45.4181.
11. Evens AM, Hong F, Gordon LI, et al. The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: a comprehensive analysis from the North American intergroup trial E2496. Br J Haematol. 2013;161(1):76-86. doi: 10.1111/bjh.12222.
12. Levis A, Anselmo AP, Ambrosetti A, et al. VEPEMB in elderly Hodgkin’s lymphoma patients. Results from an Intergruppo Italiano Linfomi (IIL) study. Ann Oncol. 2004;15(1):123-128.
13. Proctor SJ, Wilkinson J, Jones G, et al. Evaluation of treatment outcome in 175 patients with Hodgkin lymphoma aged 60 years or over: the SHIELD study. Blood. 2012;119(25):6005-6015. doi: 10.1182/blood-2011-12-396556
14. Zallio F, Tamiazzo S, Monagheddu C, et al. Reduced intensity VEPEMB regimen compared with standard ABVD in elderly Hodgkin lymphoma patients: results from a randomized trial on behalf of the Fondazione Italiana Linfomi (FIL). Br J Haematol. 2016;172(6):879-88. doi: 10.1111/bjh.13904.
15. Böll B, Bredenfeld H, Gorgen H, et al. Phase 2 study of PVAG (prednisone, vinblastine, doxorubicin, gemcitabine) in elderly patients with early unfavorable or advanced-stage Hodgkin lymphoma. Blood. 2011;118(24):6292-6298. doi: 10.1182/blood-2011-07-368167.
16. Halbsguth TV, Nogova L, Mueller H,et al. Phase 2 study of BACOPP (bleomycin, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone) in older patients with Hodgkin lymphoma: a report from the German Hodgkin Study Group (GHSG). Blood. 2010;116(12):2026-2032. doi: 10.1182/blood-2009-11-253211.
17. Martin WG, Ristow KM, Habermann TM, Colgan JP, Witzig TE, Ansell SM. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin’s lymphoma. J Clin Oncol. 2005;23(30):7614-7620.
18. Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120(2):617-624.
19. Böll B, Goergen H, Behringer K, et al. Bleomycin in older early-stage favorable Hodgkin lymphoma patients: analysis of the German Hodgkin Study Group (GHSG) HD10 and HD13 trials. Blood. 2016;127(18):2189-2192. doi: 10.1182/blood-2015-11-681064.
20. Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183-2189. doi: 10.1200/JCO.2011.38.0410.
21. Forero-Torres A, Holkova B, Goldschmidt J, et al. Phase 2 study of frontline brentuximab vedotin monotherapy in Hodgkin lymphoma patients aged 60 years and older. Blood. 2015;126(26):2798-2804. doi: 10.1182/blood-2015-06-644336.
22. Yasenchak CA, Forero-Torres A, Cline-Burkhardt VJM, et al. Brentuximab vedotin in combination with dacarbazine or bendamustine for frontline treatmetn of Hodgkin lymphom ain patients aged 60 years and above: interim results of a multi-cohort phase 2 study. American Society of Hematology, 57th Annual Meeting and Exposition; December 7, 2015, 2015; Orlando, FL.
23. Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051-1054.
24. Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065-2071.
25. Boll B, Goergen H, Arndt N, et al. Relapsed hodgkin lymphoma in older patients: a comprehensive analysis from the German hodgkin study group. J Clin Oncol. 2013;31(35):4431-4437.
26. Gopal AK, Bartlett NL, Forero-Torres A, et al. Brentuximab vedotin in patients aged 60 years or older with relapsed or refractory CD30-positive lymphomas: a retrospective evaluation of safety and efficacy. Leuk Lymphoma. 2014;55(10):2328-2334. doi: 10.3109/10428194.2013.876496.
27. Moskowitz CH, Ribrag V, Michot JM, et al. PD-1 Blockade with the Monoclonal Antibody Pembrolizumab (MK-3475) in Patients with Classical Hodgkin Lymphoma after Brentuximab Vedotin Failure: Prelimiary Results from a Phase 1b Study (KEYNOTE-013). Blood. 2014;124(21). Abstract 290.
28. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. doi: 10.1056/NEJMoa1411087.
29. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521-2532. doi: 10.1056/NEJMoa1503093.
30. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320-330.
31. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1412082.
32. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643.
33. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33(13):1430-1437. doi: 10.1200/JCO.2014.59.0703.
34. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803-1813. doi: 10.1056/NEJMoa1510665.
35. Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374(26):2542-52. doi: 10.1056/NEJMoa1603702.
36. Hamaker ME, Schiphorst AH, ten Bokkel Huinink D, Schaar C, van Munster BC. The effect of a geriatric evaluation on treatment decisions for older cancer patients a systematic review. Acta Oncol. 2014;53(3):289-296. doi: 10.3109/0284186X.2013.840741.
37. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi: 10.1200/JCO.2011.34.7625.
38. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. doi: 10.1002/cncr.26646.
39. Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy--a systematic review. Leuk Res. 2014;38(3):275-283. doi: 10.1016/j.leukres.2013.12.018.
40. Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104(9):1998-2005.
41. Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29(10):1290-1296. doi: 10.1200/JCO.2010.30.6985
42. Williams GR, Deal AM, Jolly TA, et al. Feasibility of geriatric assessment in community oncology clinics. J Geriatr Oncol. 2014;5(3):245-251. doi: 10.1016/j.jgo.2014.03.001
43. Aaldriks AA, Giltay EJ, Nortier JW, et al. Prognostic significance of geriatric assessment in combination with laboratory parameters in elderly patients with aggressive non-Hodgkin lymphoma. Leuk Lymphoma. 2015;56(4):927-935. doi: 10.3109/10428194.2014.935364.
44. Winkelmann N, Petersen I, Kiehntopf M, Fricke HJ, Hochhaus A, Wedding U. Results of comprehensive geriatric assessment effect survival in patients with malignant lymphoma. J Cancer Res Clin Oncol. 2011;137(4):733-8. doi: 10.1007/s00432-010-0933-5.
45. Zallio F, Tamiazzo S, Monagheddu C, et al. Reduced intensity VEPEMB regimen compared with standard ABVD in elderly Hodgkin lymphoma patients: results from a randomized trial on behalf of the Fondazione Italiana Linfomi (FIL). Br J Haematol. 2016;172(6):879-888. doi: 10.1111/bjh.13904.
46. Magnuson A, Allore H, Cohen HJ, et al. Geriatric assessment with management in cancer care: Current evidence and potential mechanisms for future research. J Geriatr Oncol. 2016;7(4):242-248. doi: 10.1016/j.jgo.2016.02.007.
47. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130.
48. Ballova V, Ruffer JU, Haverkamp H, et al. A prospectively randomized trial carried out by the German Hodgkin study group (GHSG) for elderly patients with advanced Hodgkin’s disease comparing BEACOPP baseline and COPP-ABVD (study HD9elderly). Ann Oncol. 2005;16(1):124-131.


