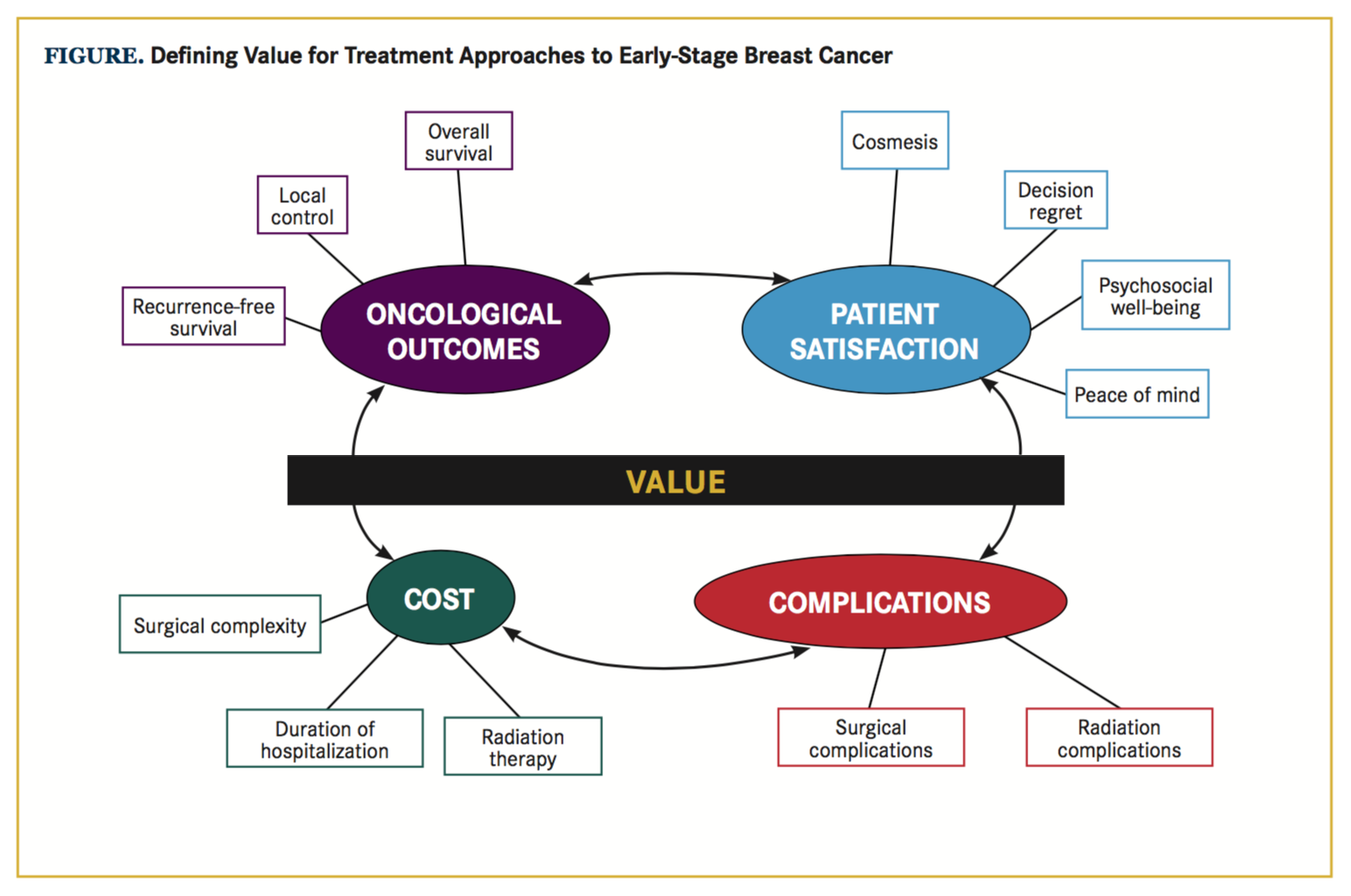
The Impetus for a Value-Based Approach
Approximately 157,000 women diagnosed this year with localized breast cancer will be presented with a wide array of guideline-concordant treatment options, including breast-conserving surgery (BCS) alone, mastectomy alone, unilateral or bilateral mastectomy with reconstruction, BCS with whole-breast irradiation, or BCS with partial-breast irradiation delivered via external-beam radiation therapy (EBRT) or brachytherapy.1,2 Over the past decade, the incidence of both unilateral and bilateral mastectomy followed by reconstruction has increased among patients who would be eligible for BCS-based interventions.3-5 For the patient and her physicians, the choice of local therapy is complex and is influenced by a multitude of factors, including access to reconstruction, desire for a favorable cosmesis, risk of complications, cost, and perception of and tolerance of recurrence risk.6,7 The challenge of choosing a therapy that reconciles many oftentimes competing factors in the setting of limited resources can be surmounted by a value-based approach to decision making. Value optimization aims to improve the full set of patient-reported and clinical outcomes without increasing total costs, or conversely, to reduce total costs without negatively impacting outcomes.8 When thought of as a ratio (Figure), patient satisfaction and oncologic outcomes occupy the numerator of the value ex- pression, while costs and complication event rates—which should be minimized—occupy the denominator.
Breast-Conserving Surgery With Radiation Is a High-Value Treatment Option
Early-stage breast cancer is the most common cancer diagnosis in women, and maximizing the value of delivered care for this disease can improve patient outcomes and reduce overall cost and healthcare resource utilization. The overall and disease-free survival equivalence of mastectomy compared with BCS followed by radiotherapy (RT) in early-stage breast cancer has been established by mature data from several large, randomized, controlled trials in North America and Europe.9-11 With excellent oncologic outcomes, increasing attention has been directed to assessing and improving value by decreasing costs, decreasing complications, and improving satisfaction with cosmesis or posttreatment quality-of-life indices.
Determining total costs is a challenging endeavor because of the fragmented nature of healthcare delivery, nonstandardized treatment approaches, and lack of consensus on what constitutes cost.8 Nevertheless, a comprehensive approach that accounts for the total costs incurred as the patient navigates the entire medical system may unmask inefficiencies or reveal the value of interventions that may be more costly upfront, but may also lead to improved outcomes or cost savings in later phases of care. For this reason, cost analysis should include all costs incurred through diagnosis, primary management, management of treatment-related complications, and follow-up.
In an analysis of 2008 Surveillance, Epidemiolo- gy, and End Results (SEER)-Medicare claims data of patients over age 65 years with localized breast cancer, mastectomy with reconstruction was estimated to cost $35,030—more than the estimated $31,388 for BCS with EBRT.12 A subsequent analysis performed several years later revealed that the overall cost difference between these 2 guideline-concordant therapies had narrowed to a difference of approximately $1750, because of an in- crease in cost of BCS with RT. Indeed, an uncomplicated course of BCS with conventional RT cost $33,500, which is approximately $1800 more than unilateral mastectomy with reconstruction.13 Unfortunately, mastectomy followed by reconstruction also has a higher relative risk (1.75, 95% CI, 1.69-1.92; P <.001) of treatment-related complications (complication rate of 66%) compared with BCS with RT (complication rate of 38%). The estimated cost of managing complications related to mastectomy with reconstruction was approximately $2700 compared with $600 for BCS with RT. In the end, when accounting for complications, the total cost of mastectomy with reconstruction exceeded that of BCS with RT. Based on the total number of patients treated, mastectomy with reconstruction resulted in a maximal global excess cost burden of approximately $2.3 million compared with BCS with hypofractionated RT.13
Cost differences between mastectomy and breast-conserving therapies appear to be magnified in the younger (<65 years), privately insured patient population. In an analysis of claims data from the MarketScan database for patients with locoregional disease, nearly 20% of patients who underwent implant or immediate reconstruction following mastectomy required treatment for infection, including hospitalization for intravenous antibiotics or additional surgical procedures, within the first 2 years of diagnosis. Autologous reconstruction was associated with a higher initial hospital stay and a higher 30-day rehospitalization risk, and implant reconstruction following mastectomy resulted in higher odds of wound complications or infections compared with nonreconstructive interventions. The estimated difference in cost due to managing complications was approximately $8600 per patient ($10,400 for mastectomy with reconstruction vs $1800 for BCS with hypofractionated RT), with an overall cost difference of $24,700 per patient.13 When considering the total number of patients treated with mastectomy and reconstruction, the maximal global excess cost burden (compared with BCS with hypofractionated RT) was approximately $88.3 million, with approximately 35% of this difference attributable to the management of complications.
High Costs and Complication Rates Currently Limit the Value of Mastectomy With Reconstruction
Many women who are involved in the decision-making process and demonstrate an understanding of anticipated recurrence and survival outcomes ultimately opt for mastectomy-based treatment options, particularly for “peace of mind.”6,14 However, patients’ satisfaction with overall cosmetic outcomes following BCS with RT has been found to compare favorably to mastectomy with either autologous or implant-based reconstruction.15,16 Despite consistently higher costs and complication rates, and equivalent patient satisfaction when compared with BCS strategies, mastectomy-based treatment approaches still may be the best option for some patients. While more than half of patients in the SEER-Medicare cohort underwent BCS with or without RT, more than half of patients in the MarketScan cohort underwent mastectomy. Among mastectomy-based treatments, mastectomy without reconstruction (estimated cost of $48,000 for MarketScan and $22,000 for SEER-Medi- care) was the most common surgical approach.13 Mastectomy alone could be considered a high-value option when considering cost, reduced treatment-related complications, and comparable disease control; however, the poor cosmetic outcome, increased risk of decision regret, and decreased patient satisfaction may decrease its overall value compared with more costly interventions such as BCS with RT and compared with mastectomy with reconstruction.17
Several other dimensions of value, such as patient satisfaction and psychosocial well-being, should also be considered when seeking to maximize value. In the setting of reconstruction, some patients report higher satisfaction with autologous compared with implant-based reconstruction. The addition of implant or autologous tissue reconstruction was associated with increased costs, with a significant component attributable to complications. Based on analysis of the MarketScan claims database, the cost of an autologous implant without complications was approximately $88,000 ($106,000 with complications), and for implant-based reconstruction the cost was $73,000 ($81,000 with complications). Choosing autologous over implant reconstruction resulted in a maximal, global cost difference of approximately $13 million, with approximately $4.8 million attributable to complications. With SEER-Medicare, the cost of an autologous implant without complications was $30,000 ($35,000 with complications) compared with $34,000 ($37,000 with complications) for implant-based reconstruction. Fewer SEER-Medicare patients receiving autologous-based reconstruction, lower reimbursement rates, and lower cost of managing complications resulted in a smaller maximal global cost difference of only $132,000.13 The improvement in patient satisfaction and sense of well-being following autologous reconstruction may justify the higher costs and wound complication risks, particularly in the older, SEER-Medicare population, where the total estimated difference when accounting for complications was $2000.15,18
Strategies for Improving Value-Based Care in Early-Stage Breast Cancer
Low-cost care is not always synonymous with higher-value care, particularly when it leads to poor oncologic outcomes. It is unclear why several hundred patients from the MarketScan database underwent BCS alone for their breast cancer, since the 2 randomized trials supporting omission of RT following BCS were limited to women older than 65 or 70 years with favorable pathologic factors. However, this is illustrative of the potential pitfall of focusing solely on cost reduction as a strategy to maximize value, as lower upfront costs may be offset by an increase in late costs, particularly in poorly selected patients who have an increased risk of recurrence without adjuvant RT. In this cohort, the total cost of proceeding with BCS alone ($70,500) exceeded BCS with conventional RT by approximately $5000 per patient when accounting for late complications, which included salvage surgery for recurrent disease.13 In the appropriately selected group of patients, omission of RT following BCS could very well be a high-value treatment option, with a low cost, decreased side-effect profile and acceptable local control rates.19,20 This is reflected in SEER-Medicare claims data, which revealed the cost of BCS alone to be $20,900—the lowest overall cost for all guideline-concordant therapies across all analyzed datasets.13 Thus, BCS alone can be an extremely high-value treatment approach, but only in a judiciously selected patient population.
BCS with RT continues to be a high-value local therapy for early-stage breast cancer, and advances in RT techniques have continued to improve the value of this therapeutic paradigm by lowering costs and improving patient-reported outcomes and treatment-related toxicities. Hypofractionated RT is an example of a treatment technique that has increased value by reducing costs and treatment-associated toxicity without compromising oncologic outcomes.21-23 In hypofractionated RT, the dose of radiation delivered per daily treatment is increased while the total number of treatments is decreased. Compared with conventional fractionated RT, which may require up to 36 daily treatments, hypofractionated RT, which can be completed in as few as 15 daily treatments, can lead to a significant reduction in healthcare expenditures, and when clinically appropriate, is estimated to reduce overall costs by an estimated $2500 to $4500 in the MarketScan and SEER-Medicare populations com- pared with conventional RT.13,24 On a global scale, if all MarketScan patients underwent hypofractionated RT, the maximal absolute cost savings based on nearly 5000 patients treated with conventional RT would be approximately $12 million for the MarketScan population and $19.5 million for the SEER-Medicare population. These are likely conservative estimates, as a National Cancer Database study estimated a potential $164 million in annual savings.25 In resource-limited settings, hypofractionated RT may also increase the number of patients who have access to RT.26
Aside from reducing the time burden for patients as well as increasing utility of treatment machines, hypofractionated RT has been associated with lower rates of acute skin toxicity and improved fatigue over conven- tional RT.22 Longitudinal assessments of patient-reported outcomes or physician-rated cosmesis have not revealed a difference between hypofractionated and conventional RT.27 Recognized as a high-value treatment option, BCS followed by hypofractionated RT may be preferred over more-aggressive surgical approaches, although both interventions are equally recommended by the National Comprehensive Cancer Network consensus guidelines for the management of early-stage breast cancer.28 Despite its demonstrated high value, adoption of BCS with hypofractionated RT in the United States has been relatively slow.29
Optimizing value is not a one-size-fits-all endeavor because patients will invariably present with different treatment goals. When formulating a treatment plan, discussions with patients should include expected longitudinal outcomes as well as the added risks of complications and increased costs in order to arrive at a high-value consensus treatment plan. These discussions can be enhanced with the help of shared decision-making tools that can provide individualized predictions of these outcomes.30,31 Future efforts should be focused on reducing complication rates by improving existing treatment techniques, formulating toxicity/complication prediction models, and investigating cost-efficient alternatives that produce equivalent oncologic outcomes.
Author affiliations: Tommy Sheu, MD; Thomas A. Buchholz, MD; and Benjamin D. Smith, MD are with the Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston; Dr Smith is also with the Department of Health Services Research, The University of Texas MD Anderson Cancer Center.
Address correspondence to: Tommy Sheu, MD, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, 1400 Pressler St, FCT 6.5000, Houston, TX 77030. Tel: (713) 792-2121. Fax: (713) 563-2545
Email: [email protected]
Author disclosures: The authors have no relevant financial relationships to disclose.
References
- National Cancer Institute. SEER Cancer Stat Facts: Female Breast Cancer. http://seer.cancer.gov/statfacts/html/breast.html. Accessed 5/30/2017.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387.
- Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nation- wide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150(1):9-16. doi: 10.1001/jamasurg.2014.2895.
- Mahmood U, Hanlon AL, Koshy M, et al. Increasing national mastectomy rates for the treatment of early stage breast cancer. Ann Surg Oncol. 2013;20(5):1436-1443. doi: 10.1245/s10434-012-2732-5.
- McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16(10):2682-2690. doi: 10.1245/s10434-009-0635-x.
- Collins ED, Moore CP, Clay KF, et al. Can women with early-stage breast cancer make an informed decision for mastectomy? J Clin Oncol. 2009;27(4):519-525. doi: 10.1200/JCO.2008.16.6215.
- Nash R, Goodman M, Lin C, et al. State variation in the receipt of a contralateral prophylactic mastectomy among women who received a diagnosis of invasive unilateral early-stage breast cancer in the United States, 2004-2012. JAMA Surg. 2017;152(7):648-657. doi: 10.1001/jamasurg.2017.0115.
- Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46-52, 54, 56-61 passim.
- Blichert-Toft M, Nielsen M, During M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol. 2008;47(4):672-681. doi: 10.1080/02841860801971439.
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233-1241. doi: 10.1056/NEJMoa022152.
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227- 1232. doi: 10.1056/NEJMoa020989.
- Shirvani SM, Jiang J, Likhacheva A, et al. Trends in local therapy utilization and cost for early-stage breast cancer in older women: implications for payment and policy reform. Int J Radiat Oncol Biol Phys. 2016;95(2):605-616. doi: 10.1016/j.ijrobp.2016.01.059.
- Smith BD, Jiang J, Shih YC, et al. Cost and complications of local therapies for early-stage breast cancer. J Natl Cancer Inst. 2017;109(1). doi: 10.1093/jnci/djw178.
- Covelli AM, Baxter NN, Fitch MI, et al. ‘Taking control of cancer’: understanding women’s choice for mastectomy. Ann Surg Oncol. 2015;22(2):383-391. doi: 10.1245/s10434-014-4033-7.
- Jagsi R, Li Y, Morrow M, et al. Patient-reported quality of life and satisfaction with cosmetic outcomes after breast conservation and mastectomy with and without reconstruction: results of a survey of breast cancer survivors. Ann Surg. 2015;261(6):1198-1206. doi: 10.1097/SLA.0000000000000908.
- Chow R, Pulenzas N, Zhang L, et al. Quality of life and symptom burden in patients with breast cancer treated with mastectomy and lumpectomy. Support Care Cancer. 2016;24(5):2191-2199. doi: 10.1007/s00520-015-3027-8.
- Lantz PM, Janz NK, Fagerlin A, et al. Satisfaction with surgery out- comes and the decision process in a population-based sample of women with breast cancer. Health Serv Res. 2005;40(3):745-767. doi: 10.1111/j.1475-6773.2005.00383.x.
- Atisha DM, Rushing CN, Samsa GP, et al. A national snap- shot of satisfaction with breast cancer procedures. Ann Surg Oncol. 2015;22(2):361-369. doi: 10.1245/s10434-014-4246-9.
- Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382-2387. doi: 10.1200/JCO.2012.45.2615.
- Kunkler IH, Williams LJ, Jack WJ, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266-273. doi: 10.1016/S1470-2045(14)71221-5.
- Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086-1094. doi: 10.1016/S1470-2045(13)70386-3.
- Shaitelman SF, Schlembach PJ, Arzu I, et al. Acute and short- term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: a randomized clinical trial. JAMA Oncol. 2015;1(7):931-941. doi: 10.1001/jamaoncol.2015.2666.
- Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513-520. doi: 10.1056/NEJMoa0906260.
- Bekelman JE, Sylwestrzak G, Barron J, et al. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008-2013. JAMA. 2014;312(23):2542-2550. doi:10.1001/jama.2014.16616.
- Greenup RA, Blitzblau RC, Houck KL, et al. Cost implications of an evidence-based approach to radiation treatment after lumpectomy for early-stage breast cancer. J Oncol Pract. 2017;13(4):e283-e290. doi: 10.1200/JOP.2016.016683.
- Khan AJ, Rafique R, Zafar W, et al. Nation-scale adoption of shorter breast radiation therapy schedules can increase survival in resource constrained economies: results from a Markov chain analysis. Int J Radiat Oncol Biol Phys. 2017;97(2):287-295. doi: 10.1016/j.ijrobp.2016.10.002.
- Swanick CW, Lei X, Shaitelman SF, et al. Longitudinal analysis of patient-reported outcomes and cosmesis in a randomized trial of conventionally fractionated versus hypofractionated whole-breast irradiation. Cancer. 2016;122(18):2886-2894. doi: 10.1002/cncr.30121.
- Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN Guide- lines Insights: Breast Cancer, Version 1.2017. J Natl Compr Canc Netw. 2017;15(4):433-451.
- Palta M, Palta P, Bhavsar NA, et al. The use of adjuvant radiotherapy in elderly patients with early-stage breast cancer: changes in practice patterns after publication of Cancer and Leukemia Group B 9343. Cancer. 2015;121(2):188-193. doi: 10.1002/cncr.28937.
- Albert JM, Liu DD, Shen Y, et al. Nomogram to predict the benefit of radiation for older patients with breast cancer treated with conservative surgery. J Clin Oncol. 2012;30(23):2837-2843. doi: 10.1200/JCO.2011.41.0076.
- Collette S, Collette L, Budiharto T, et al. Predictors of the risk of fibrosis at 10 years after breast conserving therapy for early breast cancer: a study based on the EORTC Trial 22881-10882 ‘boost versus no boost’. Eur J Cancer. 2008;44(17):2587-2599. doi: 10.1016/j.ejca.2008.07.032.