Introduction
Borderline breast epithelial lesions include atypical ductal hyperplasia (ADH), atypical lobular hyperplasia(ALH), and lobular carcinoma in situ (LCIS). These lesions are premalignant and associated with an increased risk of subsequent breast carcinoma in both the ipsilateral and contralateral breast. However, they are inconsistently treated in spite of effective chemoprevention strategies.
Early studies of borderline epithelial lesions reported these changes in approximately 4% of unselected benign breast biopsies.1 More recently, studies have reported higher frequencies of borderline epithelial lesions, ranging from 8% to 10%2,3 to 23%4; however, the last-cited study also reported a low rate for subsequent development of either ipsilateral or contralateral carcinomas,4 which raises questions about pathology diagnostic criteria.
Here, we review the pertinent pathological features, cumulative risk, and risk modifiers for subsequent invasive breast carcinoma, as well as clinical management of patients with these atypical lesions.
Histolopathological Features
Although LCIS was first reported by Foote and Stewart,5 Page and colleagues are most responsible for both carefully characterizing the histopathology of atypical breast proliferative lesions, including LCIS, and defining the subsequent risk of breast cancer following such diagnoses.1,6 According to Page, proliferative epithelial breast lesions represent a continuous spectrum of disorders consisting of benign proliferative disease, atypical proliferative lesions, carcinoma in situ, and, finally, invasive carcinomas. Histological criteria are the standard for diagnosing these lesions. Although the 2 ends of the spectrum (benign and invasive carcinoma) are histologically distinct, there is some overlap among atypical and in situ lesions.
Atypical Lobular Hyperplasia
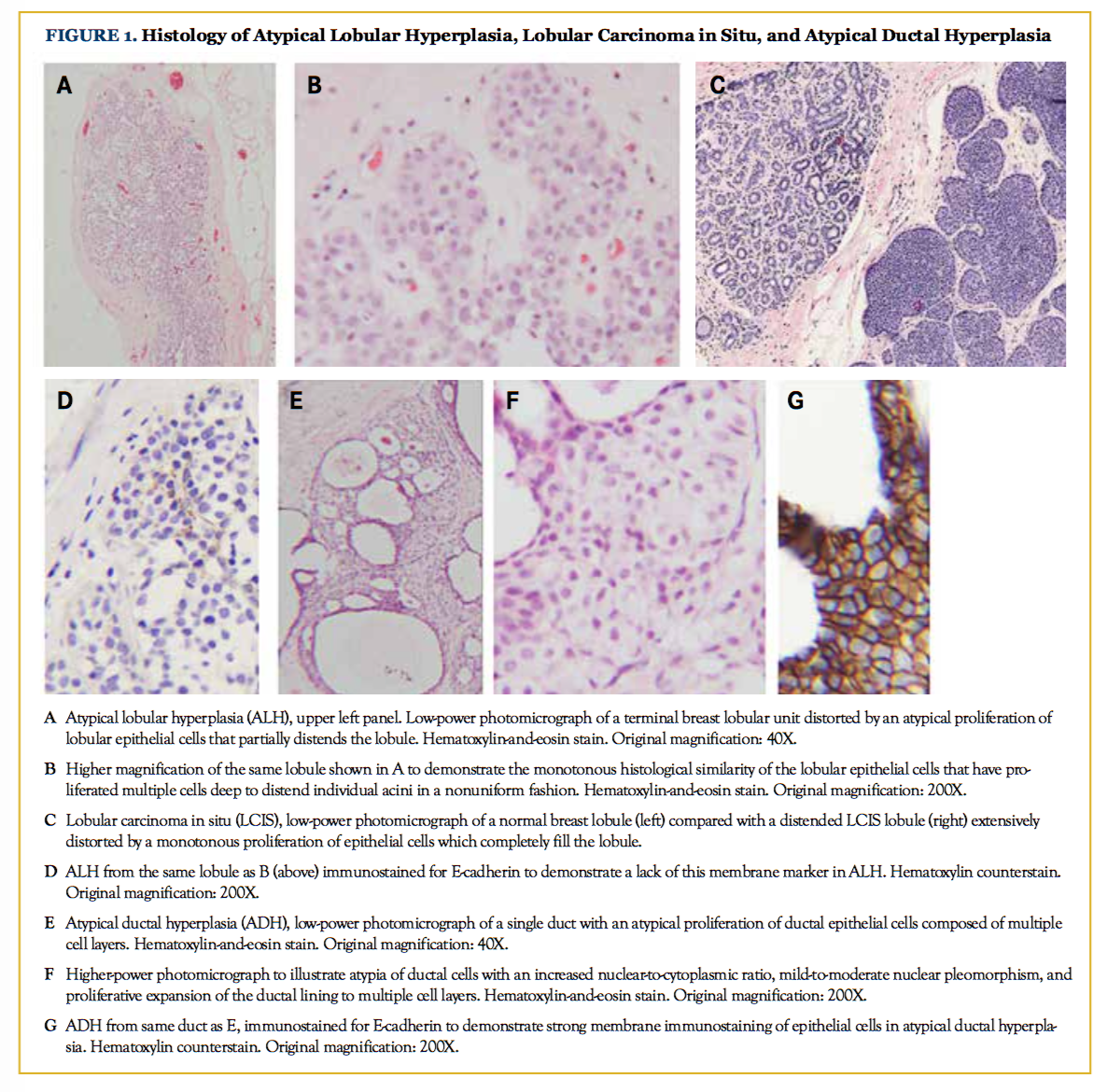
Atypical epithelial proliferations were traditionally defined as lesions that failed to meet all diagnostic criteria required for in situ carcinoma. However, these atypical borderline lesions have well-defined histological features. Atypical lobular hyperplasia (ALH) is characterized by the presence of evenly spaced round, cuboidal, or polygonal cells with hyperchromatic, round, and monotonous nuclei minimally distending or distorting the acini in the terminal duct lobular unit. These cells are noncohesive, may have small residual lumens, and occupy less than half of the acini in the lobular unit (Figures 1A and 1B).6,7
Lobular Carcinoma in Situ
Lobular carcinoma in situ (LCIS) is distinguished from ALH by the extent of disease in the lobular unit, such that there is expansion or distortion of the acini with more than half of the acini in the lobular unit filled by the atypical cells, and there is a complete absence of intracellular lumina (Figure 1C).5,7 LCIS cells remain within the terminal ductal lobular unit, or may involve adjacent ducts by pagetoid spread.8
“Classical” LCIS has been recently distinguished from “pleomorphic” LCIS (pLCIS). pLCIS may resemble a high-grade ductal neoplasm, with pleomorphic nuclei, irregular nuclear membranes, variably prominent nucleoli, and/or necrosis. Clinical experience with pLCIS is limited, and current treatment guidelines recommend management comparable with that for ductal carcinoma in situ (DCIS), ie, complete surgical resection.9
Atypical Ductal Hyperplasia
ADH is characterized histologically according to architectural criteria, cytological features, and the size of the neoplastic process. Architecturally, ADH exhibits partial involvement of the basement membrane-bound space by monotonous cells with round hyperchromatic nuclei, pale cytoplasm, and well-defined cell membranes. These cells are evenly arranged around geometric, rigid spaces, or may have micropapillae. The cytology of ADH is considered low grade, and these lesions lack atypical mitoses. ADH is said to involve fewer than 2 separate duct spaces, or is overall less than 2 to 3 mm in maximum dimension.10 To qualify as ADH, the atypical cells should form an entire nontapering bar crossing a space, or comprise a cell population of 6 or 7 cells across (Figures 1E and 1F).11
Immunostains in the Diagnosis of Atypical Proliferative Breast Lesions
Immunohistochemical stains can be used as a diagnostic aid when histopathology is not definitive. Neoplastic proliferations can be separated from benign lesions using high-molecular weight cytokeratin CK5/6 and estrogen receptor (ER) immunostains. Luminal epithelial proliferations in the benign category show widespread staining with CK5/6, and variable staining with ER; luminal neoplastic cells, however, lose CK5/6 staining in a majority of cells, and may be diffusely positive for ER.12 Further, lobular neoplasia can be distinguished from ductal lesions by a lack of E-cadherin immunohistochemical staining in the former (compare Figure 1D with 1G), provided that mutations in the CDH1 gene are not present as is seen in hereditary diffuse gastric cancer.
Molecular Characteristics
Contemporary thinking related to breast carcinogenesis involves 2 competing theories: a proliferative precursor pathway and a parallel evolution model. LCIS or DCIS has been found to be clonally related to synchronous malignant lesions approximately 40% of the time.13 Given the remarkable heterogeneity of breast cancers and premalignant lesions revealed by next-generation sequencing, mathematical models of carcinogenesis suggest that parallel evolution from preinvasive stages to subsequent malignancies may occur in some cases, suggesting very early clonal divergence in which various lesions (preinvasive or invasive) may diverge over time.14
Many studies that involve loss of heterozygosity have shown a loss of 16q in ADH, low-grade DCIS, and low-grade invasive ductal carcinoma, demon strating molecular overlap between these entities. Loss of 16q is also observed in ALH, LCIS, and invasive lobular lesions, providing evidence that these lesions share common genetic pathways with lowgrade ductal lesions.15 Lobular neoplasia is distinct from low-grade ductal lesions, as the vast majority of lobular tumors also lack E-cadherin expression because of genetic and/or epigenetic changes in the CDH1 gene.16 Molecularly, the E-cadherin gene is a tumor suppressor gene that follows Knudson’s classical 2-hit hypothesis in lobular carcinomas.17
High-grade carcinomas, including highgrade DCIS and high-grade invasive ductal carcinoma, have a distinct evolutionary pathway with HER2/neu amplification, and/or loss or gain at multiple foci including loss of 8p, 11q, 13q, 14q; gain of 1q, 5p, 8q, 17q; and amplifications on 6q22, 8q22, 11q13, 17q12, 17q22-24, and 20q13.18 Biomarkers associated with aggressive features, such as HER2 gene amplification and overexpression, TP53 mutations, and basal cytokeratins, are usually absent in lobular neoplasia.17 Classical invasive lobular carcinoma is almost universally strongly estrogen receptor–positive, thus leading to intrinsic subtyping in the luminal A subgroup by expression array classification.19 Clinically, this implies that these lesions respond to endocrine therapy or chemoprevention by hormonal therapy.
Reproducibility of Histological Diagnosis
Because borderline proliferative epithelial lesions of the breast are associated with significantly increased risks of subsequent development of invasive breast carcinomas, as summarized below, accurate diagnosis and treatment of patients with these changes is important. However, diagnosis is dependent on the ability of pathologists to consistently identify these alterations.
How accurate, then, are pathologists at making these diagnoses? Over the last few decades, many studies have evaluated pathologists’ ability to accurately classify atypical or borderline epithelial breast lesions (Table).20-25 Most of these studies show that there is a high level of agreement, and sometimes almost perfect agreement, among pathologists in diagnosing benign epithelial proliferations as well as invasive carcinomas. However, limited agreement is found in diagnosing atypical or borderline epithelial lesions of the breast.
One of the earliest studies in this regard was performed by Juan Rosai in 1991. In this study, 5 expert breast pathologists independently reviewed a selection of 17 cases with epithelial hyperplasia, and although there was an overall 58% agreement rate, the results showed that a unanimous consensus diagnosis was not attained in any of the 17 cases.22 However, this study was limited by small sample size, lack of uniform criteria for diagnosing atypical lesions, and selection of particularly challenging cases.A subsequent study by Schnitt et al showed that the interobserver agreement was higher when diagnosing atypical epithelial lesions of the breast using standardized criteria for atypical lesions.23 A large United Kingdom study also showed lack of significant agreement in the diagnosis of atypical lesions.24 Wells et al conducted a study of nonexpert (community) pathologists utilizing 30 randomly selected breast cases, not skewed towards atypical lesions, and found high overall diagnostic agreement among pathologists for most cases, but significant disagreement for atypical lesions and in situ malignancies.25 A recent study by Elmore et al21 investigated histological diagnoses of breast lesions with an emphasis on distinguishing atypical proliferative lesions from benign epithelial proliferations and carcinoma in situ among 115 experienced pathologists of varying breast diagnostic expertise in 8 different states in the United States. Higher discordance rates were found among breast biopsies from women who had higher breast density on mammographic examination, and among pathologists who interpreted lower weekly case volumes and/or worked in nonacademic settings. Also, the majority of the discordant cases were identified as difficult or requiring a second opinion by the participating pathologists themselves, suggesting that outside of the constraints of study participation, they would have sought a second opinion.21
It should be noted that none of these studies accurately reflect the process by which a pathologist renders a diagnosis for a case. None of these studies allowed examination of additional sections of the biopsy, nor were any immunohistochemical stains used; the participants were also not permitted to consult other pathologists.26 Rigid diagnostic categories were provided, and the pathologist chose the most suitable category. The majority of these studies were skewed toward challenging cases, and had an enrichment of atypical epithelial hyperplasia; this was in contrast to routine practice, in which atypical lesions are relatively infrequent (approximately 8%) among unselected cases.
Cumulative Risk of Breast Cancer After a Diagnosis of Atypical Hyperplasia
Atypical hyperplasia and LCIS are considered premalignant precursor lesions with a high risk of breast cancer in the ipsilateral breast, and an elevated risk for breast cancer bilaterally. The pioneering work in this field was conducted by Dupont and Page, who performed a retrospective study of more than 3000 women with a mean follow-up of 17 years. They found that women with atypical ductal or lobular hyperplasia had a 4.4 times greater risk of subsequent breast cancer compared with those who had a benign breast biopsy.1 Other studies have confirmed this significantly increased risk.27-29 ALH and ADH do not behave differently and are both associated with a similarly increased risk of both invasive ductal and invasive lobular carcinomas.6,30
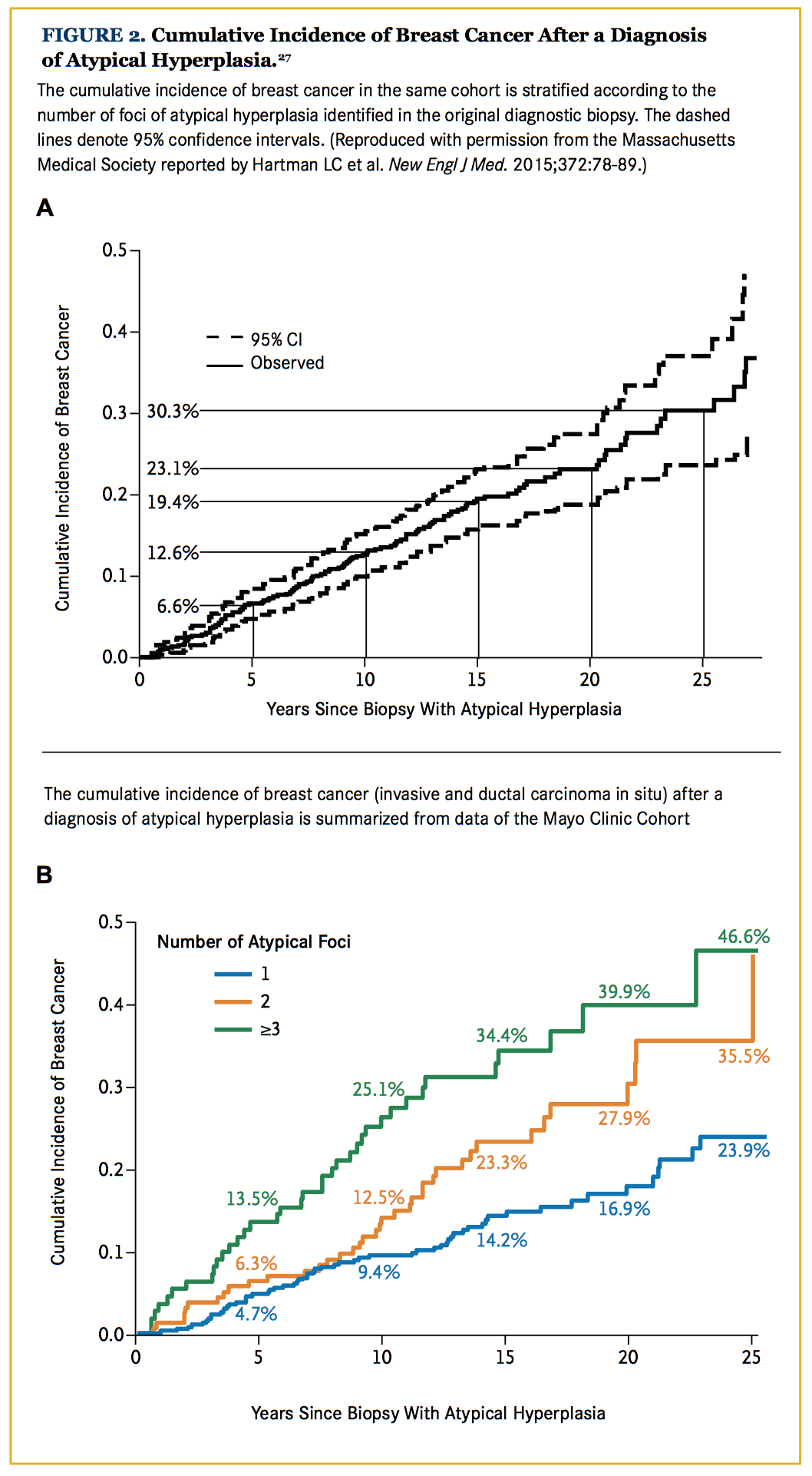
Other unfavorable histological characteristics that elevate the risk for subsequent cancers are increased by the presence of multifocal breast atypia and a younger age at diagnosis (Figure 2).29 The extent of lobular involution in the breast tissue surrounding the atypia is also inversely related to the risk of breast carcinoma development.31 The mean time from atypia diagnosis to carcinoma is 10.3 years.28
Both ADH and ALH progress to ductal carcinoma in the majority of cases, with lobular carcinomas or other histologies observed in approximately 20% to 25% of the cases after either ALH or ADH. Results of a recent study showed that 30% of all patients with atypical epithelial hyperplasia developed invasive or in situ carcinoma within 25 years of initial diagnosis. When ADH did progress to carcinoma, it progressed to ductal carcinomas in 78% of the cases, and to lobular or other breast cancer in the remaining 22%. Similarly, when ALH progressed to carcinoma, it developed into ductal carcinomas in 77% of specimens, and into lobular or other breast cancer in the remaining 23%.27,28
Women with LCIS have a 7-to 10-fold increase in breast cancer risk.30,32 The risk is found to be higher when LCIS was diagnosed before 40 years of age as compared with an initial diagnosis of LCIS after the age of 55 years.27,33 Although approximately 75% of subsequent breast cancers are ipsilateral,6,30 LCIS also predisposes to increased risk of development of carcinoma in the contralateral breast.34 LCIS may progress to either invasive ductal or invasive lobular morphology.6,34
Based on molecular as well as morphological studies, it was postulated that borderline breast lesions are precursors for low-grade DCIS and low-grade invasive carcinomas; however, recent studies have shown that these borderline lesions may progress to higher-grade cancers as well.28
Current Management
Surgical excision is recommended for women who are diagnosed with ADH (National Comprehensive Cancer Network), because 15% to 30% of the lesions initially diagnosed as ADH are found to harbor in situ or invasive carcinoma on subsequent excision. This is most likely due to the small volume of tissue sampled by core needle biopsy compared with excision biopsy.35 Other possible causes for this are that the lesion was underdiagnosed on the original biopsy, or there may have been progression to cancer from the atypical lesion, or the cancer and atypical lesion may be causing the mammographic abnormality leading to the hyperplasia being resected.27,36,37 Risk factors associated with upgrading of ADH to carcinoma include the presence of ipsilateral breast symptoms, mammographic lesions other than microcalcifications alone, small size of the needle used for core biopsy, the presence of severe ADH, papilloma codiagnosis, and a diagnosis of ADH performed by pathologists with lower practice volume; all of these were independently associated with malignancy.38 The presence of more than 3 foci involved by ADH also increases the chance of upgrading the lesion to carcinoma in the excision biopsy.39 Given that the excision of ADH may represent overtreatment, a recent series of ADH diagnosed in breast core biopsies evaluated factors predicting low risk of upgrade; the conclusion was that lack of necrosis, and either 1 ADH focus with at least 50% removal or 2 to 3 ADH foci with at least 90% removal may identify patients at low risk for whom chemoprevention without excision could be considered, if core needle biopsy results are validated prospectively.40
Clinical management of women with lobular neoplasia, which encompasses both ALH and LCIS, is controversial. Some recommend watchful waiting without surgical excision after a core breast biopsy with lobular neoplasia,41 while others recommend surgical treatment. These borderline lesions usually have a low rate of upgrade to an invasive carcinoma in an excision biopsy specimen, provided these are either incidental findings or there is concordance between radiological and pathological findings.37,42 Worrisome features in lobular neoplasia include the presence of a mass lesion or architectural distortion,8,30 nonclassical LCIS morphology, or the absence of radiologic-pathologic correlation.43,31 The National Surgical Adjuvant Breast and Bowel Project tamoxifen prevention trial demonstrated a nearly 50% reduction in subsequent development of invasive breast cancer among patients with LCIS who were treated with tamoxifen compared with placebo.44 Similar45 or greater46 risk reductions occur with raloxifene or aromatase inhibitors with less toxicity. Patients receiving surveillance, either with or without chemoprevention, should have screening mammography and clinical examination every 6 to 12 months. While pLCIS is infrequent and experience is limited, some recommend treatment similar to that for DCIS, with surgical excision but no adjuvant radiation therapy.47
Many risk-assessment models are used clinically to estimate a woman’s risk for development of carcinoma after breast biopsy. The Breast Cancer Risk Assessment Tool or the Gail model are commonly used; however, validation studies have shown that these models underestimate the risk of cancer in patients with atypical hyperplasia.48 Other models such as the International Breast Cancer Intervention Study (Tyrer-Cuzick) Model for risk assessment in women with atypical hyperplasia have also not been found to provide an accurate assessment of risk.49 Hence, it has been recommended that cumulative incidence data that portray actual breast cancer events be used to counsel women with atypical hyperplasia.49
Follow-up with annual mammography is recommended in patients with diagnosis of atypical hyperplasia. Also, the American Cancer Society guidelines recommend the use of annual MRI in addition to mammography in women who are high risk for cancer development, designated as greater than a 20% lifetime risk.50 Patients with a diagnosis of atypical hyperplasia of the breast have a higher risk and an argument has been made that these patients be similarly screened. MRI has been found to be more sensitive than mammography alone in premenopausal women with high breast density.51
The Breast Cancer Prevention Trial showed that selective estrogen receptor modulator (SERM) treatment, such as tamoxifene citrate, provides a significant risk reduction of carcinoma in women with LCIS (56%) and atypical hyperplasia (86%).52 These observations of risk reduction are supported by meta-analyses of multiple clinical trials using a variety of antiestrogen therapies (Figure 3).27,53 Tamoxifen has been shown to have a better risk-benefit profile than raloxifene, and it is more effective in reducing the risk of invasive as well as noninvasive carcinoma.54 Tamoxifen is the only drug that is recommended for chemoprevention in premenopausal women. For postmenopausal women, recent publications favor aromatase inhibitors as the preferred preventive therapy.55
Prevention Strategies
Many risk factors for breast cancer such as obesity, alcohol consumption, inactivity, and hormone replacement therapy are identified, and all but the last are on the rise. Other factors such as early menarche, late-age pregnancies, absence of breast feeding, and late menopause further increase the risk for breast cancer. Hence, weight reduction, active lifestyle, and regular physical activity, as well as decreasing alcohol consumption, have all been shown to reduce breast cancer risk.
Conclusions
There is a lack of uniformity in diagnosing and treating borderline breast lesions. Use of stringent criteria can improve the diagnostic reproducibility of borderline lesions of the breast. Surgical excision is recommended for ADH, while regular follow-up is recommended for lobular neoplasia.
The current risk prediction models are not suitable for counseling women regarding their risk of developing cancer after a diagnosis of atypical breast lesions and, therefore, reference to observed outcomes should be used. Lifestyle modifications, and annual screening by mammography with or without MRI, are recommended for women diagnosed with borderline breast lesions. Chemoprevention using SERMs or aromatase inhibitors can substantially decrease the risk of cancer development for these women.
This work was supported in part by grants from the Breast Cancer Research Foundation, the Tower Cancer Research Foundation (Jessica M. Berman Senior Investigator Award), and a gift from Dr Richard Blach. The authors would like to thank Ivonne Villalobos for her assistance with the preparation of the manuscript and figures as well as the submission of the manuscript.
Author affiliations: All authors are with the Norris Comprehensive Cancer Center, University of Southern California (USC), Los Angeles. They are all also with USC’s Keck School of Medicine, in these departments: Saloni Walia, MD, Yanling Ma, MD, Michael F. Press, MD, PhD, Department of Pathology and Laboratory Medicine; Janice Lu, MD, PhD, Department of Medicine; Julie E. Lang, MD, Department of Surgery.
Address Correspondence to:Michael F. Press, MD, PhD, Norris Comprehensive Cancer Center, NOR 5409, 1441 Eastlake Avenue, Los Angeles, CA 90033. Telephone: (323) 865-0563; E-mail: [email protected].
Final disclosures: Michael F. Press, MD, PhD, has disclosed re- search funding to his institution for work conducted in his laboratory from Cepheid, Inc; Eli Lilly & Company; Novartis Pharmaceuticals; and F. Hoffmann La-Roche, Ltd. He has served as a consultant for these companies as well as for Karyopharm Therapeutics; Puma Biotechnology; Halozyme Therapeutics; ADC Therapeutics; and Biocartis. Julie E. Lang, MD, has received research funding from ANGLE Parsortix and is a member of the speaker bureau for Genomic Health. The other coauthors have no financial disclosures or potential conflicts of interest.


References
- Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146-151.
- Pearlman MD, Griffin JL. Benign breast disease. Obstet Gynecol.2010;116(3):747-758. doi: 10.1097/AOG.0b013e3181ee9fc7.
- Vierkant RA, Degnim AC, Radisky DC, et al. Mammographic breast density and risk of breast cancer in women with atypical hyperplasia: an observational cohort study from the Mayo Clinic Benign Breast Disease (BBD) cohort. BMC Cancer. 2017;17(1):84. doi: 10.1186/s12885-017-3082-2.
- de Mascarel I, MacGrogan G, Mathoulin-Pélissier S, et al. Epithelial atypia in biopsies performed for microcalcifications. practical considerations about 2,833 serially sectioned surgical biopsies with a long follow-up. Virchows Arch. 2007;451(1):1-10.
- Foote FW, Stewart FW. Lobular carcinoma in situ: a rare form of mammary cancer. Am J Pathol. 1941;17(4):491-496.3.
- Page DL, Schuyler PA, Dupont WD, et al. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study. Lancet. 2003;361(9352):125-129. >
- Schnitt SJ, Collins LC. Biopsy Interpretation of the Breast. Philadelphia, PA: Lippincott, Williams & Wilkins; 2009.
- Middleton LP, Grant S, Stephens T, et al. Lobular carcinoma in situ diagnosed by core needle biopsy: when should it be excised? Mod Pathol. 2003;16(2):120-129.
- Flanagan MR, Rendi MH, Calhoun KE, et al. Pleomorphic lobular carcinoma in situ: radiologic-pathologic features and clinical management. Ann Surg Oncol. 2015;22(13):4263-4269. doi: 10.1245/ s10434-015-4552-x.
- Pinder SE, Ellis IO. The diagnosis and management of pre-invasive breast disease: ductal carcinoma in situ (DCIS) and atypical ductal hyperplasia (ADH)—current definitions and classification. Breast Cancer Res. 2003;5(5):254-257.
- Page DL, Rogers LW. Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. Hum Pathol. 1992;23(10):1095-1097.
- Otterbach F, Bànkfalvi A, Bergner S, et al. Cytokeratin 5/6 immunohistochemistry assists the differential diagnosis of atypical proliferations of the breast. Histopathology. 2000;37(3):232-240.
- Andrade VP, Ostrovnaya I, Seshan VE, et al. Clonal relatedness between lobular carcinoma in situ and synchronous malignant lesions. Breast Cancer Res. 2012;14(4):R103. doi: 10.1186/bcr3222.
- Sontag L, Axelrod DE. Evaluation of pathways for progression of heterogeneous breast tumors. J Theor Biol. 2005;232(2):179-189.
- Bombonati A, Sgroi DC. The molecular pathology of breast cancer progression. J Pathol. 2011;223(2):307-317. doi: 10.1002/path.2808.
- Simpson PT, Reis-Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol. 2005(2):248-254.
- McCart Reed AE, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and ’omics. Breast Cancer Res. 2015;17:12. doi: 10.1186/s13058-015-0519-x.
- Wagner PL, Kitabayashi N, Chen YT, Shin SJ. Clonal relation- ship between closely approximated low-grade ductal and lobular lesions in the breast: a molecular study of 10 cases. Am J Clin Pathol. 2009;132(6):871-876. doi: 10.1309/AJCP7AK1VWFNMCSW.
- Ciriello G, Gatza ML, Beck AH, et al; TCGA Research Net- work, Perou CM. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163(2):506-519. doi: 10.1016/j. cell.2015.09.033. >
- Beck JS. Observer variability in reporting of breast lesions. J Clin Pathol. 1985;38(12):1358-1365.
- Elmore JG, Longton GM, Carney PA, et al. Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA. 2015;313(11):1122-1132. doi: 10.1001/jama.2015.1405.
- Rosai J. Borderline epithelial lesions of the breast. Am J Surg Pathol. 1991;15(3):209-221.
- Schnitt SJ, Connolly JL, Tavassoli FA, et al. Interobserver reproducibility in the diagnosis of ductal proliferative breast lesions using standardized criteria. Am J Surg Pathol. 1992;16(12):1133-1143.
- Sloane JP, Ellman R, Anderson TJ, et al. Consistency of histopathological reporting of breast lesions detected by screening: findings of the U.K. National External Quality Assessment (EQA) Scheme. U. K. National Coordinating Group for Breast Screening Pathology. Eur J Cancer. 1994;30A(10):1414-1419.
- Wells WA, Carney PA, Eliassen MS, et al. Statewide study of diagnostic agreement in breast pathology. J Natl Cancer Inst. 1998;90(2):142-145.
- Davidson NE, Rimm DL. Expertise vs evidence in assessment of breast biopsies: an atypical science. JAMA. 2015;313(11):1109-1110. doi: 10.1001/jama.2015.1945.
- Hartmann LC, Degnim AC, Santen RJ, et al. Atypical hyperpla- sia of the breast--risk assessment and management options. N Engl J Med. 2015;372(1):78-89. doi: 10.1056/NEJMsr1407164.
- Hartmann LC, Radisky DC, Frost MH, et al. Understanding the premalignant potential of atypical hyperplasia through its natural history: a longitudinal cohort study. Cancer Prev Res (Phila). 2014;7(2):211-217. doi: 10.1158/1940-6207.CAPR-13-0222.
- Degnim AC, Visscher DW, Berman HK, et al. Stratification of breast cancer risk in women with atypia: a Mayo cohort study. J Clin Oncol. 2007;25(19):2671-2677.
- Page DL, Kidd TE Jr, Dupont WD, et al. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum Pathol. 1991;22(12):1232-1239.
- Radisky DC, Visscher DW, Frank RD, et al. Natural history of age-related lobular involution and impact on breast cancer risk. Breast Cancer Res Treat. 2016;155(3):423-430. doi: 10.1007/s10549- 016-3691-5.
- Rosen PP, Kosloff C, Lieberman PH, et al. Lobular carcinoma in situ of the breast. detailed analysis of 99 patients with average follow-up of 24 years. Am J Surg Pathol. 1978;2(3):225-251.
- Bodian CA, Perzin KH, Lattes R. Lobular neoplasia. long term risk of breast cancer and relation to other factors. Cancer. 1996;78(5):1024-1034.
- Claus EB, Stowe M, Carter D, Holford T. The risk of a contralateral breast cancer among women diagnosed with ductal and lobular breast carcinoma in situ: data from the Connecticut Tumor Registry. Breast. 2003;12(6):451-456.
- Liberman L, Dershaw DD, Glassman JR, et al. Analysis of cancers not diagnosed at stereotactic core breast biopsy. Radiology. 1997;203(1):151-157.
- Cohen MA. Cancer upgrades at excisional biopsy after diagnosis of atypical lobular hyperplasia or lobular carcinoma in situ at core-needle biopsy: some reasons why. Radiology. 2004;231(3):617-621.
- Shah-Khan MG, Geiger XJ, Reynolds C, et al. Long-term follow-up of lobular neoplasia (atypical lobular hyperplasia/lobular carcinoma in situ) diagnosed on core needle biopsy. Ann Surg Oncol. 2012;19(10):3131-3138. doi: 10.1245/s10434-012-2534-9.
- Deshaies I, Provencher L, Jacob S, et al. Factors associated with upgrading to malignancy at surgery of atypical ductal hyperplasia diagnosed on core biopsy. Breast. 2011;20(1):50-55. doi: 10.1016/j. breast.2010.06.004.
- Kohr JR, Eby PR, Allison KH, et al. Risk of upgrade of atypical ductal hyperplasia after stereotactic breast biopsy: effects of number of foci and complete removal of calcifications. Radiology. 2010;255(3):723-730. doi: 10.1148/radiol.09091406.
- Peña A, Shah SS, Fazzio RT, et al. Multivariate model to identify women at low risk of cancer upgrade after a core needle biopsy diagnosis of atypical ductal hyperplasia. Breast Cancer Res Treat. 2017;164(2):295-304. doi: 10.1007/s10549-017-4253-1.
- Dion L, Racin A, Brousse S, et al. Atypical epithelial hyper- plasia of the breast: state of the art. Expert Rev Anticancer Ther. 2016;16(9):943-953. doi: 10.1080/14737140.2016.1204916.
- Rendi MH, Dintzis SM, Lehman CD, et al. Lobular in-situ neoplasia on breast core needle biopsy: imaging indication and pathologic extent can identify which patients require excisional biopsy. Ann Surg Oncol. 2012;19(3):914-921. doi: 10.1245/s10434- 011-2034-3.
- Murray MP, Luedtke C, Liberman L, et al. Classic lobular carcinoma in situ and atypical lobular hyperplasia at percutaneous breast core biopsy: outcomes of prospective excision. Cancer. 2013;119(5):1073-1079. doi: 10.1002/cncr.27841.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst.1998;90(18):1371-1388.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial [published correction appears in JAMA. 2006;296(24):2926]. JAMA. 2006;295(23):2727-2741.
- Goss PE, Ingle JN, Alés-Martinez JE, et al; NCIC CTG MAP.3 Study Investigators. Exemestane for breast-cancer prevention in postmenopausal women [published correction appears in N Engl J Med. 2011;365(14):1361]. N Engl J Med. 2011;364(25):2381-2391. doi: 10.1056/NEJMoa1103507.
- Jorns J, Sabel MS, Pang JC. Lobular neoplasia: morphology and management. Arch Pathol Lab Med.2014;138(10):1344-1349. doi: 10.5858/arpa.2014-0278-CC.
- Pankratz VS, Hartmann LC, Degnim AC, et al. Assessment of the accuracy of the Gail model in women with atypical hyperplasia. J Clin Oncol. 2008;26(33):5374-5379. doi: 10.1200/ JCO.2007.14.8833.
- Boughey JC, Hartmann LC, Anderson SS, et al. Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J Clin Oncol. 2010;28(22):3591-3596. doi: 10.1200/ JCO.2010.28.0784.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75-89.
- Ehsani S, Strigel RM, Pettke E, et al. Screening magnetic resonance imaging recommendations and outcomes in patients at high risk for breast cancer. Breast J. 2015;21(3):246-253. doi: 10.1111/tbj.12396.
- Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351(9114):1451-1467.
- Cuzick J, Sestak I, Bonanni B, et al; SERM Chemoprevention of Breast Cancer Overview Group. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827-1834. doi: 10.1016/S0140-6736(13)60140-3.
- Visvanathan K, Hurley P, Bantug E, et al. Use of pharmaco- logic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline [published correction appears in J Clin Oncol. 2013;31(34):4383]. J Clin Oncol. 2013;31(23):2942-2962. doi: 10.1200/JCO.2013.49.3122.
- Sestak I, Cuzick J. Update on breast cancer risk prediction and prevention. Curr Opin Obstet Gynecol. 2015;27(1):92-97. doi: 10.1097/GCO.0000000000000153.





