Introduction
Monoclonal gammopathies represent a spectrum of clonal plasma cell disorders that form monoclonal gammopathy of undetermined significance (MGUS) through active multiple myeloma (MM) that are characterized by production of mono- clonal immunoglobulin heavy and/or light chains.1-3 In these disorders, detection of free light chains (FLCs) has been an evolving and, at times, critical analysis for diagnosis, prognosis, and management of treatment.4-8Historically, the standard for evaluating monoclonal light chain production has been the measurement of urine excretion of light chains determined by collecting a 24-hour urine speci- men, measuring 24-hour urine total protein, and extrapolating the quantity of light chains using data from a urine protein electrophoresis (UPEP) and immunofixation electrophoresis (IFE).1 In 2001, Freelite was developing the utilization of immu- nonephelometric technology that allowed for improved detec- tion of serum FLCs.9 Sensitivity of UPEP is 500 to 2000 mg/L,
IFE is 150 to 500 mg/L, and Freelite is 1.5 to 3.0 mg/L.10 It was then demonstrated that serum Freelite alone detected more plasma cell disorders than older methods,7 with the higher detection rate likely due to the increased sensitivity and ability to measure the ratio of abnormal to normal light chains.
Serum FLCs utilizing Freelite is now integrated into the standard of care in diagnosis and monitoring of plasma cell disorders. However, this should not replace obtaining urine IFE, as a small number of cases were missed when FLCs were not also measured.1,6 It has been our practice to use Freelite to measure the quantity of FLCs in single urine samples instead of obtaining a 24-hour urine sample with total protein and IFE. However, this has not been recommended for routine screening due to paucity of data using this assay for urinary measurements, and due to the concern for possibly confound- ing variable excretion of FLCs because of changes in renal function and variable renal reabsorption and degradation of light chains.10
The goal of this study was to evaluate the utility of using Freelite for measurement of spot urine FLCs.
Methods
Results
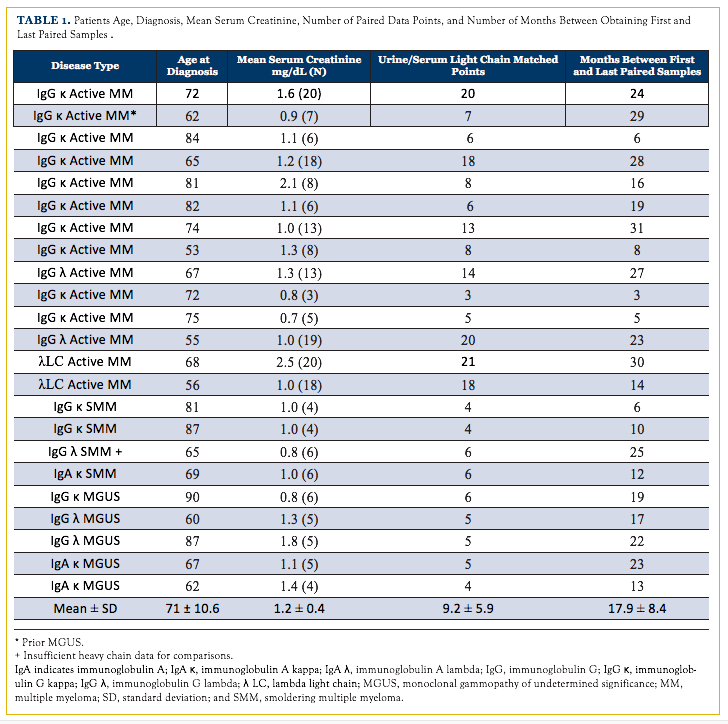
Results of comparisons between immunoglobulin parameters are illustrated in Table 2. Overall, the urine FLCs correlat- ed with serum FLCs in 9 (39%) individuals, including both patients with light-chain MM. Both urine and serum FLCs cor- related with heavy chain in 5 (25%) patients. Serum FLCs, but not urine FLCs, correlated with serum heavy chain in 6 (30%). Urine FLCs, but not serum FLCs, correlated with heavy chain in 2 (10%). There was no correlation between urine or serum FLCs with serum heavy chain in 7 (35%) patients. Use of FLC ratio instead of FLCs and “normalization” of urine FLCs based on concurrent urine and serum creatinine determinations did not change any of the correlations in any of the 23 participants (data not shown).
Figure 1 illustrates comparisons between urine and serum FLCs with serum heavy chains in 4 patients. Panels A and
B illustrate patients for whom both urine and serum FLCs correlate with serum heavy chain; Panel C illustrates an individual for whom only the serum FLCs correlated with the serum heavy chain; and Panel D illustrates an individual for whom only the urine FLCs correlated with the serum heavy chain. Figure 2 illustrates 2 patients for whom the urine FLCs correlated with the serum FLCs.
Discussion
Due to its greater sensitivity in the detection of monoclonal immunoglobulins, serum FLCs have become important for diagnosis, prognosis, and management of plasma-cell disor- ders. This method is incorporated into current guidelines for assessing patients with monoclonal gammopathies. However, measurement of urine FLCs continues to be required because a small number of individuals are detected only by measurement of urine FLC excretion.1,6
Methodology for measuring urine FLCs continues to be collecting 24-hour urine specimen and extrapolating the quantity of light chains excreted based on urine IFE.1,4 This process is subject to error when collection is incomplete, and the sensitivity of IFE is about 2-log less than the Freelite assay. Use of Freelite for measurement of urine FLCs has not been promoted because of a paucity of data using this method, and because of concern regarding using a spot urine due to variabil- ity in renal function and tubular handling of light chains,10,13 with prior studies failing to show association between urine FLCs and serum FLCs.4,8,13 We also failed to show association with aggregated data. However, there was a clear association in 39% of patients between the serum and urine FLCs, and clear associations in 25% of patients between urine FLCs and serum heavy chain. We did not specifically compare head-to-head measurement of spot urine FLCs using Freelite with 24-hour collection quantitation via IFE; however, we feel that with the 2-log increased sensitivity of Freelite, Freelite will outperform IFE. We also observed a small but defined minority of patients for whom urine FLCs outperformed serum FLCs.
Use of Freelite as an assay for immunoglobulin light chains does not substitute for urinalysis to screen for albuminuria, which may be an indication of renal light-chain amyloid. If proteinuria is indicated on the screening urinalysis, a 24-hour urine collection should be considered to determine the quantity of albumin as a part of an evaluation for amyloid disease.
We feel that due to the increased (2-log) sensitivity of Freelite over IFE, the ability to quantitate the kappa-to-lambda light chain ratio as an indication of clonality, and the convenience of a spot urine over a 24-hour collection, this assay is a valid alternative to the currently promoted methodology.
Conclusion
The measurement of urine FLCs from a single urine collection using the Freelite assay is a convenient and valid tool, and ina minority of patients with monoclonal gammopathies, it adds unique information about disease activity.
Author affiliations: Montgomery Lobe, MD, is from the Department of Medicine, Albany Medical College, Albany, NY; Donald Pasquale, MD, is from the Medical VA Care Line, Stratton VA Medical Center, Albany.
Author disclosures: The authors report no relevant conflicts of interest.
Address correspondence to: Donald Pasquale, MD, Medical VA Care Line, Stratton VA Medical Center, 113 Holland Avenue, Albany, New York 12208; email: [email protected]. Author contributions: Dr. Pasquale designed the study. Dr. Lobe collected data. Drs. Pasquale and Lobe analyzed the data and wrote the manuscript.
Supported by: Department of Veterans Affairs


References
- Dimopoulos M, Kyle R, Fermand JP, et al; on behalf of the International Myeloma Workshop Consensus Panel 3. Consen- sus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117:4701-4705. doi: 10.1182/blood-2010-10-299529.
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046-1060.
- Weiss BM, Abadie J, Verma P, et al. A monoclonal gam- mopathy precedes multiple myeloma in most patients. Blood. 2009;113(22):5412-5417. doi: 10.1182/blood-2008-12-195008.
- Bradwell AR, Carr-Smith HD, Mead GP, et al. Serum test for assessment of patients with Bence Jones myeloma. Lancet. 2003;361(9356):489-491.
- Bradwell AR. Serum free light chain measurements move to center stage. Clin Chem. 2005;51(5):805-807.
- Dispenzieri A, Kyle R, Merlini, G, et al; International Myelo- ma Working Group. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Hematology. 2009;23(2):215-224.
- Katzmann JA, Dispenzieri A, Kyle RA, et al. Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc. 2006;81(12):1575-1578.
- Nowrousian MR, Brandhorst D, Sammet C, et al. (2005) Serum free light chain analysis and urine immunofixation elec- trophoresis in patients with multiple myeloma. Clin Cancer Res. 2005;11(24 pt 1):8706-8714.
- Bradwell AR, Carr-Smith HD, Meed GP, et al. Highly sen- sitive automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47(4):673-680.
- Bradwell AR. Biology of immunoglobulin light chains. In: Serum Free Light Chain Analysis (Plus Hevylite). 6th edition. Birmingham, UK: The Binding Site Group Ltd.; 2010.
- Bruning J, Kintz BL. Computational Handbook of Statistics. 4th edition. Reading, MA: Addison Wesley Longman, Inc; 1997.
- Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19), 5019- 5032. doi: http://dx.doi.org/10.1182/blood-2011-01-293050.
- Le Bricon T, Bengoufa D, Benlakehal M, et al. Urinary free light chain analysis by the Freelite immunoassay: a preliminary study in multiple myeloma. Clin Biochem. 2002;35(7):565-567.


