Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide.1 Over the past decade, the development of molecularly targeted therapies coupled with the emergence of immunotherapy has reshaped our approach to the treatment of advanced non-small cell lung cancer (NSCLC). In addition, novel insights into the critical role of angiogenesis in NSCLC development and progression have led to the development of multiple antiangiogenic strategies. These include agents targeting the vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) pathway, a key mediator of both normal and pathologic angiogenesis implicated in tumor survival, migration, and mobilization.2-6 This review focuses on the recent developments of antiangiogenic therapies in NSCLC, which fall broadly into 2 categories: neutralizing monoclonal antibodies (mAbs) and small-molecule tyrosine kinase inhibitors (TKIs).
Monoclonal Antibodies
Bevacizumab: Patients With Treatment-Naïve NSCLC
Several mAbs targeting VEGF and VEGFR have been clinically evaluated, including bevacizumab, and more recently, ramucirumab. Bevacizumab, a humanized mAb to VEGF, interferes with VEGF binding to VEGFR.7 It is the most extensively evaluated agent in advanced lung cancer and is currently FDA-approved for use in combination with chemotherapy for patients with advanced nonsquamous NSCLC. Although reviewing all studies evaluating bevacizumab in NSCLC is outside the scope of this review, several important phase III studies warrant discussion (Table 1).
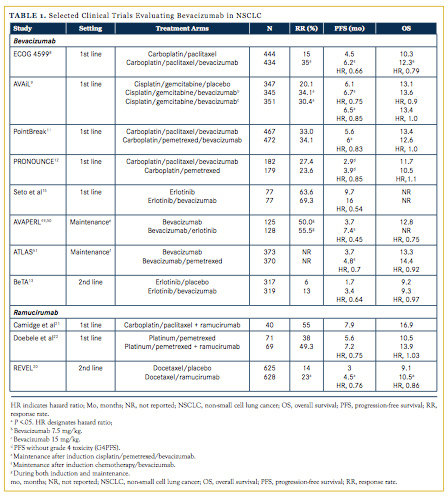
Two phase III studies, Eastern Cooperative Oncology Group (ECOG) 45998 and the AVAiL trial,9 evaluated the addition of bevacizumab to platinum-based chemotherapy versus platinum-based chemotherapy alone. The ECOG 4599 study evaluated the addition of bevacizumab to platinum chemotherapy followed by maintenance bevacizumab. In this trial, the addition of bevacizumab (15 mg/kg) to carboplatin/paclitaxel improved response rate (RR; 35% vs 15%; P <.001), progression-free survival (PFS; 6.2 vs 4.5 months; P <.01), and overall survival (OS; 12.3 vs 10.3 months; hazard ratio [HR], 0.79; P <.01) compared with carboplatin/paclitaxel alone.8 A post-hoc subset analysis of the 602 patients with adenocarcinoma from this trial demonstrated a more pronounced survival advantage with bevacizumab (14.2 vs 10.3 months; HR, 0.69; 95% CI, 0.58-0.83).10 Grade 3-5 adverse events (AEs) that were more pronounced in the bevacizumab arm included hemorrhage (4.7% vs 1.1%), hypertension (7.7% vs 0.7%), and proteinuria (3.1% vs 0%). There also were 15 treatment-related deaths in the chemotherapy-plus-bevacizumab group, including 5 from pulmonary hemorrhage, versus 2 treatment-related deaths in the chemotherapy-alone group.
Following the ECOG 4599 study, the European AVAiL trial9 evaluated the addition of bevacizumab at 2 dosages (7.5 mg/kg or 15 mg/kg) to cisplatin/gemcitabine versus cisplatin/gemcitabine alone. Whereas this study did demonstrate an improvement in PFS for both the low-dose bevacizumab group (6.1 vs 6.7 months; HR, 0.75; P = .003) and the high-dose group (6.1 vs 6.5 months; HR, 0.82; P = .03), there was no improvement in OS with either low-dose or high-dose bevacizumab (13.1, 13.4, and 13.6 months for the placebo, high-dose bevacizumab, and low dose-bevacizumab groups, respectively; HR, 1.03; P = .761). Grade 3-5 AEs that were more prevalent in the bevacizumab arms included bleeding (4% in both the low- and high-dose arms vs 2% in the placebo arm), hypertension (6% and 9% in the low- and high-dose arms, respectively, vs 2% in the placebo arm), and hemoptysis (7% and 9.7% in the low- and high-dose arms, respectively, vs 5.2% in the placebo arm).
Several trials evaluating bevacizumab in combination with platinum/pemetrexed for patients with nonsquamous NSCLC have also been completed. Two of these studies, PointBreak11 and PRONOUNCE,12 directly compared pemetrexed-containing regimens to the ECOG 4599 reference regimen of carboplatin/ paclitaxel/bevacizumab. The PointBreak study randomized patients to either induction carboplatin/paclitaxel/bevacizumab followed by maintenance bevacizumab or to carboplatin/ pemetrexed/bevacizumab followed by maintenance pemetrexed/ bevacizumab.11 Maintenance was delivered for those patients with nonprogressive disease after induction therapy. Unfortunately, this study failed to demonstrate a survival advantage with the carboplatin/pemetrexed/bevacizumab (12.6 vs 13.4 months; HR, 1.0; P = .949). Both regimens were well tolerated but resulted in different toxicity profiles, with more grade 3-4 anemia (14.5% vs 2.7%), thrombocytopenia (23.3% vs 5.6%), and fatigue (10.9% vs 5.0%) in the pemetrexed arm and more grade 3 or 4 neutropenia (40.6% vs 25.8%), febrile neutropenia (4.1% vs 1.4%), neuropathy (4.1% vs 0%), and alopecia (grade 1 or 2; 36.8% vs 6.6%) in the paclitaxel-containing arm.
The PRONOUNCE study randomized patients to either carboplatin/paclitaxel/bevacizumab induction followed by maintenance bevacizumab (CbPacBev) or to carboplatin/ pemetrexed followed by maintenance pemetrexed (CbPem).12 Similar to the PointBreak study, maintenance was given only in the absence of progressive disease in the induction regimen. The primary endpoint of PFS without grade 4 toxicity (G4PFS) was not met (3.91 for CbPem and 2.86 for CbPacBev; HR, 0.85; 90% CI, 0.7-1.04; P = .176). Although this study was not powered for OS, there also was no difference in survival between the 2 regimens, suggesting that a non-bevacizumab, pemetrexed-containing regimen may be as efficacious as the ECOG 4599 regimen.
In the second-line setting, the BeTa trial13 compared the addition of bevacizumab to erlotinib versus erlotinib plus placebo in unselected patients who had progressed on frontline therapy. Although there was an improvement in RR (13% vs 6%) and PFS (3.4 vs 1.7 months; HR, 0.62; 95% CI, 0.52-0.75) in favor of the bevacizumab arm, there was no significant survival benefit (9.3 vs 9.2 months; HR, 0.97; P = .76). Interestingly, a post-study biomarker analysis showed that the addition of bevacizumab to erlotinib had a more pronounced effect in the subgroup of patients who harbored EGFR mutations.14
Given the potential predictive utility of EGFR mutations for bevacizumab treatment, a recent Japanese study was conducted to address the role of bevacizumab in combination with erlotinib in patients with advanced-stage, treatment-naïve disease with activating EGFR mutations.15 This randomized phase II study demonstrated that the addition of bevacizumab to erlotinib significantly improved PFS compared with erlotinib alone (16 vs 9.6 months, respectively; HR, 0.54; P = .0015). At the time of publication, OS data were immature and had not been reported. The randomized phase II study evaluating this combination regimen in patients with EGFR-mutant lung cancer is being conducted in the United States.16
Ramucirumab
Ramucirumab is the fully humanized mAb that targets VEGFR2, which binds to the VEGF ligand. VEGFR2 is considered to be the most important receptor of the VEGFR family and mediates the majority of VEGF downstream signaling effects.17-19 Ramucirumab has been evaluated in both treatment-naïve and refractory NSCLC.
The phase III REVEL study20 evaluated the addition of ramucirumab to docetaxel in patients with NSCLC who had progressed after platinum-based frontline therapy. A total of 1253 patients were randomized 1:1 to docetaxel plus ramucirumab or docetaxel plus placebo. Of note, all histologies (squamous and nonsquamous) and patients with prior bevacizumab exposure were included. Similar to ECOG 4599, this study demonstrated an improvement in RR (23% vs 14%; P <.0001), PFS (4.5 vs 3.0 months; HR, 0.76; P <.0001), and OS (10.5 vs 9.1 months; HR, 0.86; P = .023) with the addition of an antiangiogenic drug to cytotoxic chemotherapy. Although not powered for subgroup analysis, improved survival was witnessed in most subsets, including patients with squamous cell histology (9.5 vs 8.2 months; HR, 0.88; 95% CI, 0.69-1.13) and responders to first-line therapy (11.2 vs 10.3 months; HR, 0.84; 95% CI, 0.71-0.99). The most common grade >3 AEs were neutropenia (49% in the ramucirumab group vs 40% in the placebo group), febrile neutropenia (16% vs 10%), and fatigue (14% vs 10%). Interestingly, the incidence of all-grade hypertension and grade >3 bleeding events were notably low in the ramucirumab arm (6% and 2%, respectively).
Two phase II studies have also evaluated the addition of ramucirumab to platinum doublet chemotherapy in the first-line setting.21,22 In a single-arm study, ramucirumab was combined with carboplatin/paclitaxel followed by ramucirumab maintenance.21 The RR and 6-month PFS were 55% and 59%, respectively. Median PFS was 7.9 months and OS was 17.9 months. Another phase II study randomizing patients to either platinum/pemetrexed or platinum/pemetrexed/ramucirumab demonstrated no significant difference in PFS (5.6 vs 7.2 months, respectively; HR, 0.75; P = .132).22 Currently, other clinical trials with ramucirumab in combination with first-line chemotherapy are ongoing, including cisplatin and gemcitabine for squamous histology and cisplatin and pemetrexed in nonsquamous NSCLC.23
Tyrosine Kinase Inhibitors
The practice-changing results from mAbs have led to the development of other antiangiogenic agents, including small-molecule TKIs targeting VEGFR. To date, several phase II and III clinical trials have been conducted evaluating multitargeted TKIs, with activity mainly directed at VEGFR2. These drugs, including vandetanib, sorafenib, sunitinib, pazopanib, nintedanib, axitinib, cediranib, and motesanib (Table 2), have been evaluated as single agents and in combination with chemotherapy or erlotinib.24-45 Although many of these studies have demonstrated modest improvements in RR and PFS, none have translated into significant survival advantages. One agent, nintedanib, warrants further discussion due to more promising activity.

Nintedanib
Cost-Effectiveness of Antiangiogenic Drugs in NSCLC
Drug costs and the definition of “value” have become important considerations when treating all cancers, including NSCLC. The cost-effectiveness of antiangiogenic drugs such as bevacizumab should be factored in when making treatment decisions. Several cost-effective analyses of bevacizumab have demonstrated the use of this drug in NSCLC to be associated with a cost of $350,000 per life-year gained.47,48
Conclusion
Over the past decade, recognition of the VEGF/VEGFR pathway as a crucial mediator of tumor survival and growth has sparked interest in the development of antiangiogenic agents for NSCLC. Several strategies have been exploited, only 2 drugs, bevacizumab and ramucirumab, have demonstrated survival advantages in patients with treatment-naïve and refractory NSCLC, respectively. Moving forward, several questions remain regarding future trials and the routine clinical use of these agents. First, the utility of additional clinical trials evaluating TKIs in unselected patient populations is unclear, given that none of these agents have demonstrated improvements in survival, thus far. The exception to this has been nintedanib, which demonstrated a survival advantage in the subset of patients with adenocarcinoma in LUME-Lung 1.
Second, no clear predictive biomarkers have been identified to guide therapy selection for this class of drugs. Because of the genomic heterogeneity of NSCLC, molecular enrichment strategies have become paramount in identifying patients eligible for targeted therapies. Given the cost and potential toxicity of these drugs, further studies to identify serum and tissue biomarkers are urgently needed. The recently reported results from the Japanese study and the ongoing US phase II ACCRU study evaluating bevacizumab in combination with erlotinib in EGFR-positive patients may define a molecular niche for this agent.
Third, it remains unclear whether bevacizumab and ramucirumab should be combined with any nontaxane cytotoxic chemotherapy. Although bevacizumab added to carboplatin/ paclitaxel demonstrated a survival advantage, this agent added no survival benefit when combined with cisplatin/gemcitabine and has never been evaluated combined with platinum/ pemetrexed compared with platinum/pemetrexed alone. Finally, the cost-effectiveness of antiangiogenic drugs should be factored in when making treatment decisions.
We look forward to future studies evaluating novel agents exploiting the VEGF pathway, and hope that further efforts will help better define which patients are more likely to benefit from such strategies.
Affiliations: Jean Lee, MD, and Daniel Becker, MD, are from New York University Langone Medical Center. Dr Becker is also from the Veterans Administration Hospital, New York, NY; Benjamin Levy, MD, is from the Icahn School of Medicine, Mount Sinai Health Systems, New York, NY.
Disclosures: Dr Levy serves as a paid consultant for Eli Lilly, Genentech, Celgene Corporation, AstraZeneca, and Biodesix; Drs Lee and Becker report no relevant financial conflicts to disclose.
Address correspondence to: Benjamin Levy, MD, W. 15th St, New York, NY 10011. Phone: 212-604-6017; fax: 212-604-6038; email: [email protected]
References
- Howlader N NA, Krapcho M, Garshell J, et al, (eds) . SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute; 2014.
- Brown LF, Berse B, Jackman RW, et al. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993;143(5):1255-1262.
- Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53(19):4727-4735.
- Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36(2):127-137.
- Mattern J, Koomagi R, Volm M. Association of vascular endothelial growth factor expression with intratumoral microvessel density and tumour cell proliferation in human epidermoid lung carcinoma. Br J Cancer. 1996;73(7):931-934.
- Senger DR, Van de Water L, Brown LF, et al. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993;12(3-4):303-324.
- Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57(20):4593-4599.
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542-2550.
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227-1234.
- Sandler A, Yi J, Dahlberg S, et al. Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5(9):1416-1423.
- Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(34):4349-4357.
- Zinner RG, Obasaju CK, Spigel DR, et al. PRONOUNCE: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients ith advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2015;10(1):134-142.
- Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377(9780):1846-1854.
- Herbst R, Stern HM, Amler LC. Biomarker evaluation in the phase III, placebo-controlled, randomized BeTa trial of bevacizumab and erlotinib for patients with advanced non-small cell lung cancer (NSCLC) after failure of standard 1st-line chemotherapy: correlation with treatment outcomes. .J Thorac Oncol. 2009;4:S323.
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15(11):1236-1244.
- Research AaCC. Erlotinib with or without bevacizumab in treating patients with stage IV non-small cell lung cancer with EGFR mutations. NLM Identifier: NCT01532089. https:// clinicaltrials.gov/ct2/show/NCT01532089. Accessed July 13, 2015.
- Youssoufian H, Hicklin DJ, Rowinsky EK. Review: monoclonal antibodies to the vascular endothelial growth factor receptor-2 in cancer therapy. Clin Cancer Res. 2007;13(18 Pt 2):5544s-5548s.
- Zeng H, Dvorak HF, Mukhopadhyay D. Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) peceptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem. 2001;276(29):26969-26979.
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368-4380.
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665-673.
- Camidge DR, Berge EM, Doebele RC, et al. A phase II, open-label study of ramucirumab in combination with paclitaxel and carboplatin as first-line therapy in patients with stage IIIB/IV non-small-cell lung cancer. J Thorac Oncol. 2014;9(10):1532-1539.
- Doebele RC, Spigel D, Tehfe M, et al. Phase 2, randomized, open-label study of ramucirumab in combination with first-line pemetrexed and platinum chemotherapy in patients with nonsquamous, advanced/metastatic non-small cell lung cancer. Cancer. 2015;121(6):883-892.
- Lilly E. A study of pemetrexed and carboplatin/cisplatin or gemcitabine and carboplatin/cisplatin with or without IMC-1121B in participants previously untreated with recurrent or advanced non-small cell lung cancer (NSCLC). NLM Identifier: NCT01160744. ClinicalTrials website. https://clinicaltrials.gov/ ct2/show/NCT01160744. Accessed July 13, 2015.
- Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(11):1835-1842.
- Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(4):650-656.
- Socinski MA, Wang XF, Baggstrom MQ, et al. Sunitinib switch maintenance in advanced non-small cell lung cancer (NSCLC): an ALLIANCE (CALGB 30607), randomized, placebo-controlled phase III trial. J Clin Oncol. 2014;32(5S; abstr 8040)
- Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial. J Clin Oncol.. 2012;30(17):2070-2078.
- Reck M, Kaiser R, Mellemgaard A, et al; LUME-Lung 1 Study Group. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15(2):143-155.
- Goss GD, Arnold A, Shepherd FA, et al. Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J Clin Oncol. 2010;28(1):49-55.
- Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11(7):619-626.
- Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(8):1059-1066.
- Lee JS, Hirsh V, Park K, et al. Vandetanib versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR). J Clin Oncol. 2012;30(10):1114-1121.
- de Boer RH, Arrieta O, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol.. 2011;29(8):1067-1074.
- Heymach JV, Paz-Ares L, De Braud F, et al. Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(33):5407-5415.
- Twelves C, Chmielowska E, Havel L, et al. Randomised phase II study of axitinib or bevacizumab combined with paclitaxel/ carboplatin as first-line therapy for patients with advanced non-small-cell lung cancer. Ann Oncol. 2014;25(1):132-138.
- Schiller JH, Larson T, Ou SH, et al. Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a phase II study. J Clin Oncol. 2009;27(23):3836-3841.
- Scagliotti GV, Vynnychenko I, Park K, et al. International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J Clin Oncol. 2012;30(23):2829-2836.
- Blumenschein GR, Jr., Kabbinavar F, Menon H, et al; Motesanib NSCLC Phase II Study Investigators. A phase II, multicenter, open-label randomized study of motesanib or bevacizumab in combination with paclitaxel and carboplatin for advanced nonsquamous non-small-cell lung cancer. Ann Oncol. 2011;22(9):2057-2067.
- Weiss JM, Villaruz LC, Socinski MA, et al. A single-arm phase II trial of pazopanib in patients with advanced non-small cell lung cancer with non-squamous histology with disease progression on bevacizumab containing therapy. Lung Cancer. 2014;86(2):288-290.
- Reck M, Kaiser R, Eschbach C, et al. A phase II double-blind study to investigate efficacy and safety of two doses of the triple angiokinase inhibitor BIBF 1120 in patients with relapsed advanced non-small-cell lung cancer. Ann Oncol. 2011;22(6):1374-1381.
- Belani CP, Yamamoto N, Bondarenko IM, et al. Randomized phase II study of pemetrexed/cisplatin with or without axitinib for non-squamous non-small-cell lung cancer. BMC Cancer. 2014;14:290.
- Spigel D, Burris HA, 3rd, Greco FA, et al. A randomized phase II study of pazopanib or placebo in combination with erlotinib in patients with advanced non-small celllung cancer. J Thorac Oncol. 2012;7(9):(Suppl abstr 13).
- Hanna NH Kaiser R, Sullivan RN, et al. LUME-Lung 2: a multicenter, randomized, double-blind, phase III study of nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with advanced nonsquamous non-small cell lung cancer (SNCLC) after failure of first-line chemotherapy. J Clin Oncol.
- Paz-Ares LG, Biesma B, Heigener D, et al; NSCLC [non– small-cell lung cancer] Research Experience Utilizing Sorafenib (NExUS) Investigators Study Group. Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol. 2012;30(25):3084-3092.
- Paz-Ares L, Hirsh V, Zhang L, et al. Monotherapy administration of sorafenib in patients with non-small cell lung cancer: phase III, randomized, double-blind, placebo-controlled MISSION trial. Presented at: the 37th ESMO Congress; September 28-October 2, 2012; Vienna, Austria. Abstract 916.
- Ingelheim B. LUME-Columbus: nintedanib plus docetaxel in advanced non-small cell lung cancer with translational research. NLM Identifier: NCT02231164. https://clinicaltrials.gov/ct2/ show/NCT02231164. Accessed July 13, 2015.
- Klein R, Muehlenbein C, Liepa AM, et al. Cost-effectiveness of pemetrexed plus cisplatin as first-line therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2009;4(11):1404-1414.
- Goulart B, Ramsey S. A trial-based assessment of the cost-utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer. Value Health. 2011;14(6):836-845.
- Barlesi F, Scherpereel A, Gorbunova V, et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous non small-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol. 2014;25(5):1044-1052.
- Barlesi F, Scherpereel A, Rittmeyer A, et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol. 2013;31(24):3004-3011.
- Johnson BE, Kabbinavar F, Fehrenbacher L, et al. ATLAS: randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2013;31(31):3926-3934.





