Introduction
Roughly 10% to 15% of lung adenocarcinomas diagnosed in the United States harbor activating mutations in the epidermal growth factor receptor (EGFR) gene.1 The remarkable efficacy of small-molecule EGFR tyrosine kinase inhibitors (TKIs) in this unique subset of patients has revolutionized the therapeutic approach to lung cancer over the past 10 years, and has created a treatment paradigm for other molecularly defined subsets of cancer. Multiple randomized phase 3 studies have demonstra-ted that EGFR TKIs are superior to chemotherapy in patients with stage IV EGFR-mutated disease, with excellent response rates (58%-75%) and, on average, a doubling of progression-free survival (PFS).2-5 Thus, agents such as erlotinib, gefitinib, or afatinib are now standard first-line therapy for advanced non-small cell lung carcinoma (NSCLC) with sensitizing EGFR mutations. More recently, the second-generation EGFR TKI afatinib also has shown a significant overall survival (OS) benefit compared with first-line chemotherapy, specifically in patients with EGFR exon 19 deletion–positive tumors.6
The unprecedented success of these agents in the metastatic setting logically leads to the clinical question: Are EGFR TKIs beneficial as adjuvant therapy for patients with earlier stages of disease? The importance of this question cannot be overstated. Although early-stage lung cancers are treated surgically with curative intent, recurrence rates after complete anatomic resection remain unacceptably high, ranging from 30% to 70%.7 Tumor recurrence is in fact the primary obstacle to long-term survival. Only 36% to 73% of patients with stage IA-IIB lung cancer are alive at 5 years; for those with stage III disease, the 2-year survival is less than 50% despite definitive therapy.8 We have learned that adjuvant/neoadjuvant cisplatin-based doublet chemotherapy can marginally improve survival by eradicating occult micrometastases. The Lung Adjuvant Cisplatin Evaluation (LACE) trial,7 a pooled analysis of 5 large trials (4584 patients), demonstrated a 5-year OS benefit of 5.4% with chemotherapy. This is a fairly modest gain considering the toxicity associated with cisplatin-based chemotherapy, and leaves us in dire need of novel adjuvant approaches to improve cure rates.
Success in Other Tumor Types
The use of molecularly targeted therapies in an adjuvant setting is not unprecedented, and there are lessons to be learned from successes in other tumor types. One example is imatinib mesylate therapy for resected gastrointestinal stromal tumors (GIST) that express constitutively activated mutant isoforms of KIT protein. In a landmark phase 3 trial of 770 patients, adjuvant imatinib demonstrated dramatically improved disease-free survival (DFS) compared with placebo for resected GIST (hazard ratio [HR] = 0.35; P <.0001).9 More recently, a randomized study showed improved OS in patients with GIST who received 3 (vs 1) years of adjuvant imatinib after resection, with survival curves remaining apart well past the point of TKI discontinuation.10 A second important example is adjuvant trastuzumab, which significantly improves OS after resection of HER2-positive breast cancer, with benefits that exceeded expectations based on its value in the metastatic setting.11 These experiences provide compelling reasons to investigate the role of targeted agents in the adjuvant management of NSCLC.
Early Clinical Trials of Adjuvant EGFR Inhibition
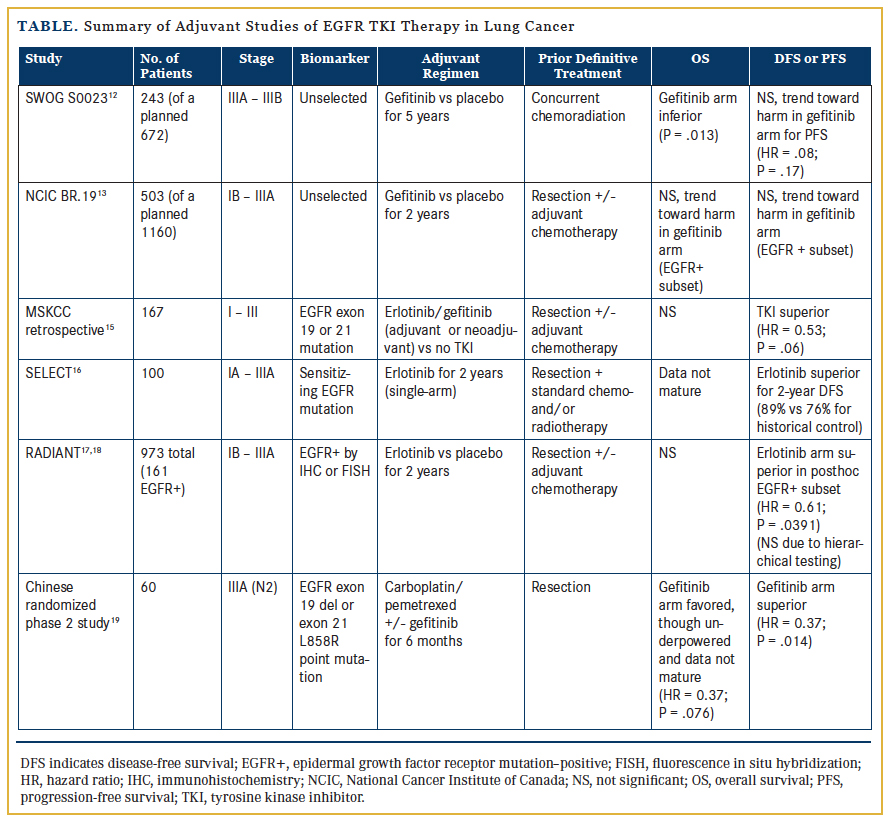
The earliest exploration of adjuvant EGFR inhibition involved 2 large randomized trials initiated over 10 years ago. Although both were negative studies, it is important to recognize that neither trial was enriched for patients with EGFR mutations. The first was SWOG S0023,12 a phase 3 study designed to enroll 672 patients with unresectable, locally advanced (stage III) NSCLC receiving definitive chemoradiation. Patients whose disease did not progress after treatment were subsequently randomized to gefitinib 250 mg per day or placebo. However, an unplanned interim analysis (of 243 patients) in 2005 demonstrated an unexpectedly inferior survival for those receiving gefitinib (median of 23 vs 35 months; P = .013). The study was thus closed prematurely, and routine use of maintenance EGFR TKIs in stage III disease is not currently recommended outside of a clinical trial.
The National Cancer Institute of Canada (NCIC) phase 3 BR.19 trial13 was the first randomized, double-blind, placebo-controlled investigation of a targeted agent (gefitinib) delivered in the adjuvant setting for completely resected NSCLC. Patients with stage IB-IIIA NSCLC were randomized, following surgical resection and optional adjuvant chemotherapy, to either 2 years of adjuvant gefitinib or placebo. Unfortunately, this study was also terminated early due to safety concerns based on the negative phase 3, placebo-controlled ISEL trial14 that demonstrated no survival benefit with gefitinib as second- or third-line treatment for metastatic disease, as well as the aforementioned S0023 interim report.12 Thus, BR.19 accrued only 503 of the planned 1160 patients, and the median duration of study therapy was less than 5 months. Only 76 patients had EGFR-mutated disease (36 in the gefitinib arm, 40 in the placebo arm). An exploratory analysis of this subgroup showed no difference in DFS (HR = 1.22; P =.15) or OS (HR = 1.24; P = .14), and a trend towards harm with gefitinib. In summary, BR.19 was underpowered, terminated early, and nonenriched for the relevant population, and had suboptimal duration of therapy. It is thus impossible to draw statistically robust conclusions from these data regarding the impact of adjuvant EGFR inhibition in early-stage NSCLC.
Promising Recent Trial Data
Although these initial studies were disappointing, more recently reported data offer promising insights. In 2011, investigators at Memorial Sloan Kettering Cancer Center (MSKCC) retrospectively reviewed a prospectively maintained surgical database of 167 patients with resected stage I-III NSCLC harboring EGFR exon 19 or 21 mutations.15 They compared 2 cohorts—one with 56 patients who received either adjuvant or neoadjuvant EGFR TKI therapy, and the other including 111 patients who did not receive TKI therapy. In the multivariate analysis, which controlled for stage and adjuvant platinum chemotherapy, the 2-year DFS was 89% for the TKI-treated cohort compared with 72% for the control group (HR = 0.53; P = .06). Importantly, however, there was no statistically significant difference in 2-year OS. The retrospective nature of this study introduces the possibility of significant bias, as treatment was primarily based on the preferences of patients and their oncologists. This highlights the crucial need for prospective trials.
The first prospective data to suggest that adjuvant targeted therapy may indeed alter the disease course for early-stage NSCLC were presented at the 2014 American Society of Clinical Oncology (ASCO) Annual Meeting, from the SELECT and RADIANT trials. The SELECT trial16 was a multicenter, single-arm, phase 2 study of adjuvant erlotinib in resected, early-stage, EGFR mutation–positive NSCLC. Patients with stage IA-IIIA disease who completed routine adjuvant chemotherapy and/or radiotherapy subsequently received erlotinib 150 mg daily for 2 years, followed by computed tomography (CT) surveillance. The investigators reported a 2-year DFS of 89%, an improvement over the historical control of 76% that was used to power the study. While this is an encouraging result, a considerable drop-off in DFS was seen by 3 years among these highly selected patients, and with the absence of a comparator arm, conclusions cannot be reached regarding true benefit. Of note, the majority of recurrences (25 of 29) were seen after erlotinib discontinuation, raising important questions about the optimal duration of TKI therapy.
Also reported at ASCO 2014 were the results of RADIANT,17 a phase 3 study investigating adjuvant erlotinib in patients with resected NSCLC with overexpression of EGFR protein by immunohistochemistry (IHC) or EGFR gene amplification by fluorescence in situ hybridization (FISH). These 2 selection biomarkers are no longer considered to be of significant value, and only 16.4% of enrolled patients had tumors with activating EGFR mutations. After complete resection (stage IB-IIIA) and optional adjuvant chemotherapy, patients were randomized (2:1) to receive either erlotinib 150 mg daily or placebo for 2 years. Posthoc subset analysis of the EGFR-mutated population (161 patients; 102 erlotinib, 59 placebo) favored erlotinib, with a median DFS of 46.4 months compared with 28.5 months with placebo (HR = 0.61; P = .0391, though not statistically significant due to hierarchical testing).18 The OS data remain immature, with only 22% of necessary events having occurred, and the median was thus not reached. RADIANT highlights some of the pitfalls of a posthoc analysis using a biomarker for which the study was not stratified, raising concerns about statistical power and validity as well as potential confounders. For example, among the patients with EGFR mutations, 30.5% in the placebo arm had stage IIIA disease compared with only 17.6% in the erlotinib arm. Because most stage III patients will recur, this major imbalance likely biases the results in favor of adjuvant therapy.
Additional prospective data come from a recently published, small, randomized phase 2 Chinese study investigating patients with resected stage IIIA (N2) NSCLC harboring EGFR mutations (exon 19 deletions or L858R point mutations).19 Sixty patients
were enrolled and received adjuvant carboplatin/pemetrexed chemotherapy, then were randomized to either gefitinib 250 mg daily for 6 months or observation. The primary endpoint was DFS, and the study was powered to show a 20% improvement after 2 years. The results were quite impressive, with a median DFS of 40 months in the gefitinib arm compared with only 27 months with placebo (HR = 0.37; P = .014). The 2-year OS was 92% versus 77%, favoring gefitinib (HR = 0.37; P = .076), although the survival data remain premature, and this small study was not powered to show OS benefit.
No Improvement in OS
Despite these strides forward, none of the adjuvant studies described in Table 1 indicate an improvement in OS—the key measure that must be demonstrated for any adjuvant therapy. This leaves us asking a crucial question: Do EGFR TKIs truly eliminate micrometastases (thereby curing the patient), or do they merely suppress minimal residual disease for a period of time? If the latter is true, one could perhaps argue in favor of reserving the targeted agent until the time of relapse, when it could then be offered as rescue therapy.
In fact, a long-standing concern about adjuvant EGFR inhibition is the notion that exposure to erlotinib or gefitinib may alter the tumor’s biology, rendering it resistant at the time of recurrence (via a secondary mutation such as T790M, or through other mechanisms). This concern was discussed in a small yet thought-provoking 2011 retrospective MSKCC study in which 22 patients with disease recurrence after adjuvant EGFR TKI therapy were identified.20 Eleven of these patients were retreated, and 8 of the 11 responded for a median duration of 10 months. Furthermore, repeat biopsies revealed the T790M resistance mutation only in patients on active therapy—those who had already completed adjuvant TKI therapy did not have tumor genotypes with secondary resistance mutations. This suggests that retreatment with erlotinib/gefitinib at the time of recurrence is feasible, and implies that longer durations of adjuvant therapy may be necessary.
Questions Remain
Many other unanswered questions remain. First, exactly which “early-stage” patients should qualify for adjuvant EGFR inhibition? Much like with adjuvant chemotherapy, the benefit derived from a TKI will be relative to the absolute risk of disease recurrence, and is thus expected to be less for those with lower stages of disease. With this basic principle in mind, an oncologist may be more inclined to offer adjuvant TKIs to patients with high-risk stage III disease than to those with relatively low-risk stage I disease. Caution must be exercised in this regard, however. As previously discussed, the SWOG S0023 experience showed that gefitinib was actually harmful after definitive chemoradiation for locally advanced disease.12 Ultimately, we lack the data necessary to construct sound treatment guidelines based on relative risk.
Second, what is the optimal duration of adjuvant TKI therapy? Most recurrences in the SELECT and RADIANT trials occurred after discontinuation of erlotinib.16,17 Although this may suggest a need for more than 2 years of treatment, the aforementioned Chinese adjuvant study demonstrated positive results with only 6 months of adjuvant gefitinib.19 An ongoing clinical trial using afatinib (NCT01746251) is evaluating 3 versus 24 months of adjuvant therapy for resected stages I-III NSCLC in an effort to determine whether prolonged treatment courses are superior to shorter ones.
Third, adverse events associated with chronic EGFR TKI therapy must also be considered. Although these agents are generally well tolerated, the side effects are certainly not negligible; rash and diarrhea (each occur in 50% of cases) can have significant impact on quality of life for some, and well-informed decisions must be made before exposing patients to these potential risks.
Finally, the cost of therapy remains a key issue. A 2-year course of erlotinib costs approximately $150,000, and there are roughly 10,000 EGFR-mutated lung cancer resections per year in the United States alone. If all these patients were to receive adjuvant erlotinib, it would amount to a staggering $1.5 billion healthcare cost. A conclusive demonstration of clinical benefit is necessary before committing to such an expenditure in the Affordable Care Act era.
Phase 3 Trials Needed
The data from RADIANT and SELECT suggest that adjuvant TKI therapy may offer a consistent and significant reduction in the risk of early recurrence for patients with EGFR-mutated disease, potentially improving upon adjuvant chemotherapy. However, as encouraging as this may seem, the data are far from conclusive, and phase 3 prospective trials remain necessary. Several such studies are under way, the most pivotal being the ALCHEMIST study, a suite of integrated precision medicine trials that aim to provide definitive answers. Powered for OS, ALCHEMIST will compare 2 years of adjuvant TKI versus placebo therapy for resected, early-stage lung adenocarcinoma, using erlotinib for EGFR-mutated or crizotinib for ALK-translocated disease. A trial of this importance should have already been completed by now. As it now stands, mature data will not be available for another 10 years—an unacceptably long time to wait.
In the meantime, our focus is quickly shifting to the more specific third-generation EGFR TKIs—agents in development such as AZD9291 and CO-1686—that have shown higher effi-cacy, more favorable side-effect profiles, and activity in advanced T790M mutation-bearing disease. These inhibitors will almost certainly be available for mainstream use well ahead of the final data analysis from ALCHEMIST. To this end, a randomized study comparing AZD9291 with placebo in the adjuvant setting is planned. We strongly support early initiation of such critical adjuvant trials with newer agents in an effort to answer this question in a far timelier manner. Only then can we take the next steps forward to continue improving cure rates for our patients with this deadly disease—namely, to determine whether EGFR inhibition can replace chemotherapy altogether in the adjuvant space, and to investigate targeted and immunotherapy combination approaches.
Conclusion
While adjuvant EGFR TKI therapy may ultimately prove to be beneficial, current data supporting its use remain limited and an OS advantage has not yet been demonstrated. Nonetheless, molecular testing for EGFR (and ALK) gene mutations should be seriously considered in patients with resected lung adenocarcinomas so that appropriate patients can be offered enrollment in ongoing adjuvant trials whenever possible. Outside of clinical studies, we must have informed and balanced discussions with our patients with EGFR-mutated NSCLC regarding adjuvant TKI therapy, carefully weighing the pros and cons in light of the currently limited available data
Affiliations: Ramsey Asmar, MD, is a fellow in Hematology and Oncology, and Balazs Halmos, MD, is associate professor of Medicine at Columbia University Medical Center, New York-Presbyterian Hospital, New York, NY.
Disclosures: Dr Halmos has received clinical research funding from Astellas, Genentech/Roche, AstraZeneca, and Boehringer Ingelheim, and has performed consulting for Genentech/Roche, AstraZeneca, Clovis, and Boehringer Ingelheim. Dr Asmar reports no relevant conflicts of interest to disclose.
Address correspondence to: Balazs Halmos, MD, Division of Hematology/Oncology, Columbia University Medical Center, 161 Fort Washington Ave, 9th Floor, New York, NY 10032; phone: 212-305-3997; fax: 646-317-6321; email: [email protected].
References
- Zhang Z, Stiegler AL, Boggon TJ, Kobayashi S, Halmos B. EGFR-mutated lung cancer: a paradigm of molecular oncology. Oncotarget. 2010;1(7):497-514.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-2388.
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121-128.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957.
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246.
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141-151.
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552-3559.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):706-714.
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9669):1097-1104.
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272.
- Smith I, Procter M, Gelber RD, et al. 2-year followup of trastuzumab after adjuvant chemotherapy in HER2- positive breast cancer: a randomised controlled trial. Lancet. 2007;369(9555):29-36.
- Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26(15):2450-2456.
- Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR.19 study. J Clin Oncol. 2013;31(27):3320- 3326.
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005;366(9496):1527-1537.
- Janjigian YY, Park BJ, Zakowski MF, et al. Impact on diseasefree survival of adjuvant erlotinib or gefitinib in patients with resected lung adenocarcinomas that harbor EGFR mutations. J Thorac Oncol. 2011;6(3):569-575.
- Pennell NA, Neal JW, Chaft JE, et al. SELECT: a multicenter phase II trial of adjuvant erlotinib in resected early-stage EGFR mutation-positive NSCLC. J Clin Oncol. 2014;32(15 suppl; abstr 7514).
- Kelly K, Altorki NK, Eberhardt WEE, et al. A randomized, double-blind phase 3 trial of adjuvant erlotinib (E) versus placebo (P) following complete tumor resection with or without adjuvant chemotherapy in patients (pts) with stage IB-IIIA EGFR positive (IHC/FISH) non-small cell lung cancer (NSCLC): RADIANT results. J Clin Oncol. 2014;32(15 suppl; abstr 7501).
- Shepherd FA, Altorki NK, Eberhardt WEE, et al. Adjuvant erlotinib (E) versus placebo (P) in non-small cell lung cancer (NSCLC) patients (pts) with tumors carrying EGFR-sensitizing mutations from the RADIANT trial. J Clin Oncol. 2014;32(15 suppl; abstr 7513).
- Li N, Ou W, Ye X, et al. Pemetrexed-carboplatin adjuvant chemotherapy with or without gefitinib in resected stage IIIA-N2 non-small cell lung cancer harbouring EGFR mutations: a randomized, phase II study. Ann Surg Oncol. 2014;21(6):2091- 2096.
- Oxnard GR, Janjigian YY, Arcila ME, et al. Maintained sensitivity to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer recurring after adjuvant erlotinib or gefitinib. Clin Cancer Res. 2011;17(19):6322-6328.