Introduction
Recognition of molecularly defined subsets of non– small cell lung cancer (NSCLC), targeted therapies, and immunotherapeutics has transformed the management of patients with advanced NSCLC. In this review, we will discuss the significance of frontline molecular testing to optimize treatment selection, testing at the time of resistance to offer effective second-line strategies, and implications of circulating tumor DNA (ctDNA) testing. Selected key and representative clinical studies permitting validation of the most relevant biomarkers will also be reviewed without the goal to provide a comprehensive overview of the treatment landscape.
Frontline Testing to Optimize Treatment Selection
ImmunotherapyThe introduction of checkpoint inhibitors has revolutionized management of advanced NSCLC. The majority of lung cancers—in particular, smoking-associated tumors—harbor a large number of missense mutations, and they are immunogenic through the presentation of neoantigens. In many lung tumors, a T-cell response is established but is negatively regulated via the tumoral expression of checkpoint molecules. PD-1 is a transmembrane receptor present on T cells, which interacts with the PD-1 ligand, PD-L1, present on multiple cell types, including tumor cells (TCs) and PD-L2. Engagement of PD-1 with the PD-L1 receptor negatively regulates T-cell function and leads to immune evasion.1 The following 3 aspects of antitumor response provide promising biomarkers to assess for potential benefit with checkpoint inhibitor therapy: 1) molecular makeup of the tumor, measured by tumor mutation burden (TMB); 2) lymphocytic infiltration of the tumor (so-called “inflamed tumor”), measured by a variety of assays; and 3) tumoral “defense” response, assessed by PD-L1 immunohistochemistry.
The anti–PD-1 antibody nivolumab was the first checkpoint inhibitor to be approved for the treatment of advanced NSCLC. In the CheckMate-017 and -057 trials, nivolumab significantly improved median overall survival (OS) compared with docetaxel in patients who experienced disease progression on platinum-based chemotherapy. There was a trend for higher levels of PD-L1 expression in the Checkmate 057 trial.2
In the pivotal KEYNOTE-010 study,3 pembrolizumab, another anti–PD-1 antibody, was found to improve OS and progression-free survival (PFS) compared with docetaxel in patients with PD-L1– positive tumors (defined as PD-L1 expression of at least 1%), with a striking improvement in the subset of patients with PD-L1 expression of at least 50%. This study led to the FDA approval of pembrolizumab in the second-line setting for PD-L1–positive (expression >1%) tumors, and it provided impetus for the biomarker-selected, first-line studies, such as the KEYNOTE-024 study.4 In this study, single-agent pembrolizumab was compared with standard doublet chemotherapy for patients with treatment-naïve, advanced NSCLC with PD-L1 tumor proportion score score of ≥50%, and significant OS benefit and less toxicity were demonstrated for the pembrolizumab group, leading to the approval of pembrolizumab as first-line treatment for advanced PD-L1–high-positive NSCLC.4
NSCLCs with high TMB are associated with better response to immune checkpoint inhibitors.5 For example, while the frontline CheckMate-026 study did not demonstrate benefit for nivolumab versus doublet chemotherapy in patients with advanced PD-L1–positive NSCLC (defined as PD-L1 expression of ≥5%),6 an unplanned subset analysis suggested that outcomes (PFS) improved in patients with tumors harboring a high TMB, suggestive of additional benefit of TMB determination in optimizing immunotherapy selection.7 It should be noted that tumors with low TMB, such as EGFR/ALK/ROS-mutated NSCLC appear to have low objective response rates (ORRs) to immunotherapy, around 4%, arguing for reserving immunotherapy until after exhausting more effective targeted and chemotherapy options for this subset of patients.
The randomized phase III OAK8 and phase II POPLAR9 studies demonstrated increased OS with the anti–PD-L1 antibody atezolizumab compared with docetaxel for the overall group of patients; furthermore, patients with tumors expressing high levels of PD-L1, as measured by a unique immunohistochemistry-based assay assessing both TC and immune cell PD-L1 expression, derived the greatest benefit. The POPLAR study intriguingly suggested that highly inflamed tumors, as assessed by a T-cell effector/ gamma interferon signature, demonstrate better response to atezolizumab, suggestive of a third potential biomarker for patient selection.9
While PD-L1 is now a validated biomarker for selecting patients for single-agent, frontline immunotherapy, several challenges should be noted:
- PD-L1 can often be heterogeneously expressed in tumor tissue.10
- Patients without PD-L1 expression can still have benefit from a checkpoint inhibitors equivalent to that of second-line chemotherapy, with fewer adverse effects; therefore, PD-L1 status should not exclude a patient from immunotherapy.2,8
- Much confusion has existed around the various PD-L1 assays using different proprietary antibodies, platforms, and scoring criteria. The Blueprint study suggested similar performance of the generally used antibodies, with the exception of the SP142 antibody that appears to show less TC staining.11
- Recent approval of the combination of doublet chemotherapy plus pembrolizumab, regardless of PD-L1 expression, based on the KEYNOTE- 021 study, provides further options, but also adds complexity as to the proper use of biomarkers for patient selection; further clarity is anticipated from completion of pivotal studies in this area.12
Targeted Therapies
Over the past decade, multiple molecularly defined subsets of patients have been identified, and appropriate genomic testing is a critical component of proper patient management.
EGFR mutations are more commonly found in nonsmokers, women, and Asians, and occur in approximately 10% to 15% of all lung adenocarcinomas in the United States.13 EGFR tyrosine kinase inhibitors (TKIs) are the standard first-line treatment for advanced NSCLC in tumors harboring EGFR mutations (Figure 1). The 2 most common EGFR mutations are exon 19 deletion and the L858R point mutation in exon 21, accounting for more than 90% of known activating EGFR mutations.14 EGFR TKIs improve ORRs and delay disease progression compared with chemotherapy in tumors harboring these mutations. The landmark IPASS trial15 was among the initial studies showing dramatic improvement in PFS with gefitinib compared with chemotherapy in patients with EGFR mutations. Several trials since have shown improved ORR and PFS with gefitinib, erlotinib, and afatinib compared with chemotherapy. For example, the OPTIMAL trial16 showed improved PFS of 13.1 versus 4.6 months, while the EURTAC study17 showed improved PFS of 9.7 versus 5.2 months compared with chemotherapy. Overall survival was not significantly different in these trials, but this is likely confounded by crossover effects.
Afatinib is a second-generation irreversible EGFR TKI, with similar improvement in PFS in the LUX-Lung 318 and LUX-Lung 619 trials, with an OS benefit noted in the subset of patients with exon 19 deletions. Results of the randomized phase II LUX-Lung 7 study20 suggested a modest but significant PFS benefit but no OS benefit of afatinib versus gefitinib in the frontline setting, whereas a randomized, phase III study of another irreversible inhibitor, dacomitinib, versus gefitinib recently was reported to show an even more impressive PFS benefit for dacomitinib.20,21
Rearrangement of the ALK gene on chromosome 2, most commonly associated with echinoderm microtubule-associated protein-like 4, and less commonly with other partners, results in a fusion oncogene found in 2% to 3% of patients with NSCLC; it is most commonly seen in younger nonsmokers and in adenocarcinomas.22 The PROFILE 1014 trial23 showed that the ALK inhibitor crizotinib was superior to standard chemotherapy as first-line therapy in patients with ALK-positive, advanced NSCLC. Alectinib and ceritinib are second-generation ALK inhibitors with improved central nervous system (CNS) penetration, and they demonstrate activity in crizotinib-refractory patients. In a recent trial, alectinib was associated with a significantly prolonged PFS and increased time to intracranial progression when used as a frontline agent compared with crizotinib, and it may become the preferred first-line treatment.24 Another similarly potent agent, brigatinib, was recently granted accelerated approval for treatment of patients with ALK-positive NSCLC who progressed on or were intolerant to crizotinib.25,26 A fifth agent, lorlatinib, is currently under investigation; it demonstrates activity in multiple subsets of highly treatment-refractory patients.
ROS proto-oncogene 1 receptor tyrosine kinase chromosomal rearrangements can be found in about 1% of patients with advanced lung adenocarcinoma. Patients with ROS1 rearrangements are more likely to be younger and never-smokers.27 Given outstanding activity noted in a phase I cohort experience, crizotinib is FDA approved and recommended as first-line therapy in ROS1-positive tumors.28
A number of other actionable alterations have emerged over the past few years. Two key MET alterations include MET exon 14 skipping mutations, occurring in 3% to 4% of NSCLCs, with enrichment in sarcomatoid lung cancers and MET amplification. Tumors harboring such alterations can respond to MET TKIs, such as crizotinib and cabozantinib.29,30 Overlap between these 2 types of alterations can occur; however, there may be a difference in clinical features associated. Most exon 14 mutations seem to occur in never-smokers; however, MET amplification has been more commonly seen in smokers. And while ORRs in patients with MET exon 14 skipping mutations have been generally very high, responses in patients with MET-amplified tumors might be more variable and dependent on level of amplification, with higher responses noted in tumors with more than 5- to 6-fold amplification.31
Activating BRAF mutations occur in about 3% of patients with lung adenocarcinoma. BRAF V600E mutations can respond to BRAF TKIs, such as vemurafenib and dabrafenib. The combination of dabrafenib with the MEK inhibitor trametinib showed an excellent ORR of 63% in a phase II study, and it has received FDA approval.32
Neurotrophic tyrosine receptor kinase (NTRK) fusions are reported in about 1% to 3% of NSCLCs. Larotrectinib is the first pan-TRK inhibitor currently in clinical development, with reportedly high ORRs across multiple tumor types harboring NTRK alterations including NSCLC, and tissue-agnostic approval is anticipated.33
RET gene fusions make up another NSCLC subset with reported response to the currently available RET inhibitors. A phase II trial of the RET inhibitor cabozantinib showed partial response in 28% of the patients.34 An international registry of patients with RET-rearranged tumors showed that multikinase inhibitors such as cabozantinib, vandetanib, and sunitinib had limited activity against these tumors.35
HER2 (ErbB2) mutations or amplifications are seen in 1% to 2% of lung adenocarcinomas. At present, the role of targeted therapies is under investigation, with case series demonstrating activity for chemotherapy/ trastuzumab combinations, but not providing clarity as to targeting of ErbB2.
A recent study reported some benefit for the use of T-DM1 for these patients, with higher responsiveness with higher amplification.36 HER2 mutations, such as recurrent insertions of exon 20 (YVMA) and extracellular point mutations (eg, S310F), differ from HER2 amplification. Novel EGFR/ErbB2 inhibitors are in development that promise targeting of this patient subset.37
Currently, genomic testing for EGFR/ALK/ROS is recommended for all patients with advanced, nonsquamous NSCLC, regardless of clinical characteristics. The National Comprehensive Cancer Network (NCCN) additionally recommends testing for BRAF, ErbB2 (HER2), MET, and RET
A recent review from 15 community oncology centers showed that EGFR and ALK testing was done in 69% and 65%, respectively, of patients with advanced NSCLC, and only 12% underwent testing for the 5 additional mutations recommended by NCCN. Although molecular testing is expensive, tedious, and time-consuming, clinical outcomes are much improved with targeted therapies for appropriate patients, which may lower the total cost of care. Therefore, they should be broadly implemented.38
Testing at the Time of Progression to Offer Effective Second-Line Strategies
Development of resistance to molecularly targeted and immunotherapy agents is a major problem. Significant advances have been made in understanding the mechanisms of resistance, and additional testing on tissue specimens or plasma is crucial to choosing the most appropriate next-line option when resistance develops (Figure 2).
Acquired resistance to EGFR TKIs is seen within 6 to 18 months of starting therapy.39 In up to 60% of cases, a secondary mutation in the EGFR gene, the T790M mutation, is the cause.40 The phase I/II AURA-1 and AURA-2 trials studied the efficacy of the third-generation EGFR TKI osimertinib in EGFR T790M-mutated cases, demonstrating ORRs of 50% to 60% and favorable toxicity profiles. The phase III AURA-3 study showed superiority of osimertinib over chemotherapy in patients with confirmed T790Mpositive, advanced NSCLC after first-line EGFR TKI therapy, with significantly improved PFS (10.1 vs 4.4 months), ORR (71% vs 31%), and better adverseeffect profile. Notably, patients with CNS disease had improved outcomes, with PFS of 8.5 months with osimertinib versus 4.2 months with chemotherapy.41 These results led to full FDA approval of osimertinib in 2017. Results of the FLAURA study42 comparing osimertinib with first-generation EGFR TKIs, such as gefitinib or erlotinib, in treatment-naïve patients with EGFR-mutated, advanced NSCLC, have recently been presented. Frontline osimertinib significantly prolonged PFS versus the comparator first-generation agents, and it also demonstrated excellent CNS activity and safety. While OS data are still forthcoming, these results are likely to change the frontline treatment landscape, and have already been incorporated into NCCN guidelines.
Other causes of resistance to EGFR TKIs include activation of bypass signaling pathways.43,44 MET amplification has been reported in about 5% of tumors at the time of resistance, and dual EGFR and MET inhibition, which is under investigation, can induce apoptosis in these cases in vitro.43,44 Phenotypic changes, namely epithelial-to-mesenchymal transition and small cell transformation, are other known mechanisms of resistance.45,46
Gainor et al studied the mechanisms of resistance in ALK-positive tumors following disease progression on first- or second-generation ALK inhibitors,47 and found ALK kinase domain mutations in 20% of crizotinib-resistant, 54% of ceritinib-resistant, 53% of alectinib-resistant, and 71% of brigatinib-resistant samples. Distinct mutations were found for each of the 3 second-generation ALK inhibitors. The G1202R solvent front mutation, which causes resistance to first- and second-generation ALK inhibitors, was found in 21% ceritinib-, 29% alectinib-, and 43% brigatinib-resistant samples. The novel ALK inhibitor lorlatinib can overcome this mutation. Compound mutations were identified in 12.5% of patients resistant to second-generation ALK inhibitors. Lorlatinib was the only agent effective on compound mutations. Epithelialto-mesenchymal transition was observed in 42% (5 of 12) of cases. Such results suggest that understanding the secondary events causing resistance to ALK inhibitors is crucial in optimizing selection of the next line of treatment, which reinforces the need for repeat biopsies.48
There is a high rate of primary resistance to immune checkpoint inhibitors, and furthermore, most patients develop acquired resistance to PD-1/PD-L1 blockade. Primary resistance can occur due to lack of PD-L1 expression on TCs, insufficient tumor-infiltrating lymphocytes, exhausted CD8+ T cells, and low TMB.5,47 Acquired resistance is common, but the mechanisms are not well understood. In melanoma, several mechanisms of resistance have been described including upregulation of alternative immune checkpoints notably T-cell immunoglobulin mucin-3, genetic aberrations that result in immune escape including loss-of-function mutations in the genes encoding interferon receptor associated Janus kinase-1 or Janus kinase-2 resulting in lack of response to interferon gamma, and mutations in the antigen-presenting protein beta-2-microglobulin leading to loss of surface expression of major histocompatibility complex I and T-cell escape.49,50 It is yet to be seen whether similar events occur in NSCLC. Recently, it has been shown that tumors with STK11 deficiency have high TMB but low PD-L1 expression and they may not respond to checkpoint inhibitors; this defines a potential primary resistance mechanism.51
The Use of ctDNA as a Powerful New Tool to Assist in Treatment Selection
Molecular classification of NSCLC has allowed for the use of effective targeted therapies for defined subsets of patients. However, the need for repeated biopsies for molecular analyses is challenging. The use of ctDNA analysis has emerged as a powerful tool that can detect actionable mutations for diagnosis and treatment monitoring.52,53 Using a validated capture-based assay, ctDNA has been detected in the plasma of 84% of patients with advanced NSCLC.54 Advantages of plasma-based ctDNA testing, besides the noninvasive nature, include the short turnaround time and better representation of tumor heterogeneity. Drawbacks are lack of ctDNA shedding in some tumors55 and lower sensitivity to detect copy number alterations and rearrangements.56 The most common methods for ctDNA detection include digital-polymerase chain reaction (dPCR) and next-generation sequencing. dPCR assays are highly sensitive and specific but can only capture a small number of mutations. Next-generation sequencing has the potential to detect multiple mutations from a single sample.56,57
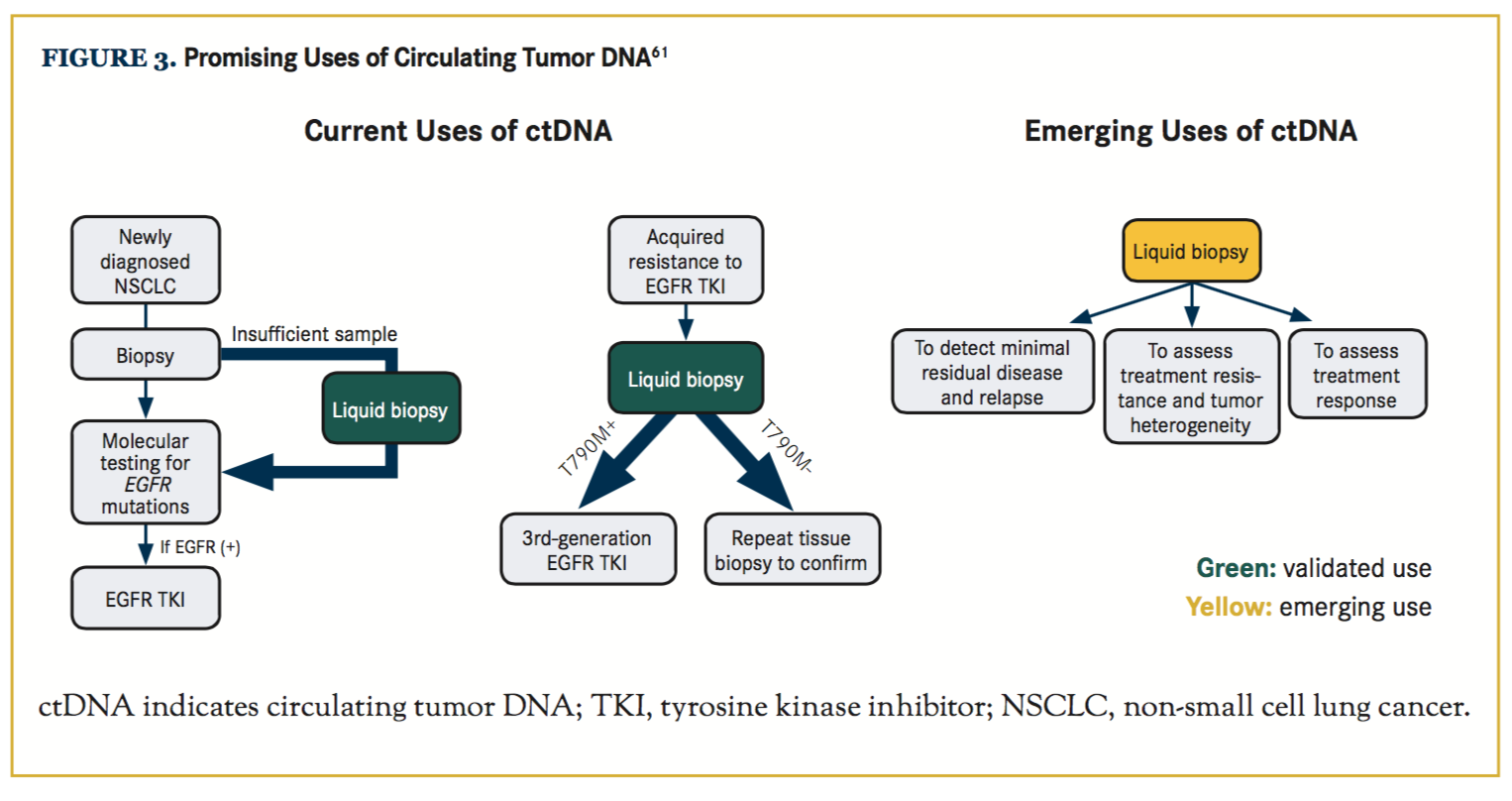
CtDNA testing was first validated in EGFR T790M testing. Sensitivity of 81.25% and 100% specificity of T790M detection by dPCR assays was reported in a study.58 In another study, the sensitivity of T790M detection by ctDNA was reported at 70%, and the ORR and median PFS with osimertinib were similar in patients with T790M-positive disease in plasma versus tumor.59 It is clear that the positive predictive value of detecting EGFR mutations in ctDNA is high enough to justify initiation ofthird-generation TKI therapy, but the negative predictive value is less robust and does not rule out the presence of an EGFR mutation, and needs to be confirmed with tissue sampling. Similar sets of studies also validated use of ctDNA for frontline EGFR mutation testing, which can be very useful in cases with limited biopsy samples. Emerging data suggest significant utility of ctDNA in detecting ALK resistance mutations as well.60 Multigene platforms are available and similarly useful for detection of alterations affecting other genes in both primary diagnosis and acquired resistance settings. Other highly promising areas of use for ctDNA testing are in detecting minimal residual disease in localized lung cancer following definitive therapy, to assist in risk stratification, in adjuvant treatment decisions, and to guide treatment re-initiation at early time points (Figure 3).61
Conclusions
Upfront molecular and immune biomarker testing is paramount for optimal patient selection with the broadening availability of highly effective immunotherapeutic and targeted agents for biomarker-selected patient subsets. Repeated testing is needed to assess mechanisms of acquired resistance and proper sequencing of effective agents. ctDNA testing may increase our ability to further improve patient outcomes and patient experience.
Author affiliations: Shirin Attarian, MD, and Balazs Halmos, MD, MS, are with the Department of Oncology, Montefiore Medical Center, Bronx, NY. Niyati Goradia, MBBS is with the Department of Medicine, Jacobi Medical Center, Bronx, NY. Benjamin P. Levy, MD, is with the Johns Hopkins Kimmel Cancer Center at Sibley Memorial Hospital, Washington, DC.
Address correspondence to: Balazs Halmos, MD, Department of Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10461. E-mail: [email protected]
Financial disclosures: The authors have no relevant financial relationships to disclose


References
- Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54(4):307-314.doi: 10.1007/s00262-004-0593-x.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550.doi: 10.1016/S0140-6736(15)01281-7.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEY- NOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi: 10.1056/NEJMoa1606774.
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124-128. doi: 10.1126/science.aaa1348.
- Carbone DP, Reck M, Paz-Ares L, et al; CHECKMATE 026 Investigators. First-line nivolumab in stage IV or recurrent non- small-cell lung cancer. N Engl J Med. 2017;376(25):2415-2426. doi: 10.1056/NEJMoa1613493.
- Peters S, Creelan B, Hellmann MD, et al. Impact of tumor mutation burden on the efficacy of first-line nivolumab in stage IV or recurrent non-small cell lung cancer: an exploratory analysis of CheckMate 026. Presented at: 2017 American Association for Cancer Research Annual Meeting; April 1-5, 2017; Washington, DC. Abstract CT082.
- Rittmeyer A, Barlesi F, Waterkamp D, et al; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X.
- Fehrenbacher L, Spira A, Ballinger M, et al; POPLAR Study Group. Atezolizumab versus docetaxel for patients with pre- viously treated non-small-cell lung cancer (POPLAR): a multi-centre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846. doi: http://dx.doi.org/10.1016/S0140-6736(16)00587-0.
- Casadevall D, Clavé S, Taus Á, et al. Heterogeneity of tumor and immune cell PD-L1 expression and lymphocyte counts in surgical NSCLC samples [published online May 4, 2017]. Clin Lung Cancer. doi: 10.1016/j.cllc.2017.04.014.
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12(2):208-222. doi: 10.1016/j.jtho.2016.11.2228.
- Langer CJ, Gadgeel SM, Borghaei H, et al; KEYNOTE-21 Investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497-1508. doi: 10.1016/S1470-2045(16)30498-3.
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Nat Cancer Inst. 2005;97(5):339- 346. doi: 10.1093/jnci/dji055.
- Riely GJ, Yu HA. EGFR: the paradigm of an oncogene-driven lung cancer. Clin Cancer Res. 2015;21(10):2221-2226.doi: 10.1158/1078-0432.CCR-14-3154.
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with ad- vanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866-2874. doi: 10.1200/JCO.2010.33.4235.
- Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemo- therapy as first-line treatment of EGFR mutation-positive ad- vanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol. 2015;26(9):1877-1883. doi: 10.1093/annonc/mdv276.
- Rosell R, Carcereny E, Gervais R, et al; Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first- line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol.2012;13(3):239-246. doi: 10.1016/S1470-2045(11)70393-X.
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334. doi: 10.1200/JCO.2012.44.2806.
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with ad- vanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213-222. doi: 10.1016/S1470-2045(13)70604-1.
- Paz-Ares L, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017;28(2):270-277. doi: 10.1093/annonc/mdw611.
- Mok T, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib for the first-line treatment of advanced EGFR mutation positive non-small cell lung cancer (ARCHER 1050): a randomized, open-label phase III trial. Presented at: 2017 American Society of Clinical Oncology Annual Meeting; Jumne 2-6, 2017; Chicago, IL. Abstract LBA9007.
- Takahashi T, Sonobe M, Kobayashi M, et al. Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol. 2010;17(3):889-897. doi: 10.1245/s10434-009-0808-7.
- Solomon BJ, Mok T, Kim DW, et al; PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer [published correction appears in N Engl J Med. 2015;373(16):1582]. N Engl J Med. 2014;371(23):2167-2177.doi: 10.1056/NEJMoa1408440.
- Peters S, Camidge DR, Shaw AT, et al; ALEX Trial Investigators. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829-838. doi: 10.1056/NEJMoa1704795.
- Camidge DR, Bazhenova L, Salgia R, et al. Safety and efficacy of brigatinib (AP26113) in advanced malignancies, including ALK+ non-small cell lung cancer (NSCLC). Presented at: 2015 American Society of Clinical Oncology Annual Meeting; May 29-June 2, 2015; Chicago, IL. Abstract 8062. http://ascopubs.org/doi/abs/10.1200/jco.2015.33.15_suppl.8062.
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non- small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490-2498. doi: 10.1200/JCO.2016.71.5904.
- Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863-870. doi: 10.1200/JCO.2011.35.6345.
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rear- ranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963- 1971. doi: 10.1056/NEJMoa1406766.
- Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015;5(8):842- 849. doi: 10.1158/2159-8290.CD-14-1467.
- Camidge DR, Ou S-HI, Shapiro G, et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC). J Clin Oncol. 2014;32(suppl 15; abstr 8001). https://meetinglibrary.asco.org/record/92507/abstract.
- Caparica R, Yen CT, Coudry R, et al. Responses to crizotinib can occur in high-level MET-amplified non-small cell lung cancer independent of MET exon 14 alterations. J Thorac Oncol.2017;12(1):141-144. doi: 10.1016/j.jtho.2016.09.116.
- Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung can- cer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17(7):984-993. doi: 10.1016/S1470-2045(16)30146-2.
- Hyman DM, Laetsch TW, Kummar S, et al. The efficacy of larotrectinib (LOXO-101), a selective tropomyosin receptor kinase (TRK) inhibitor, in adult and pediatric TRK fusion cancers. J Clin Oncol. 2017;35(suppl; abstr LBA2501). http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.18_suppl. LBA2501?af=R.
- Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung can- cer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17(12):1653-1660. doi: 10.1016/S1470-2045(16)30562-9.
- Gautschi O, Milia J, Filleron T, et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol. 2017;35(13):1403- 1410. doi: 10.1200/JCO.2016.70.9352.
- Gatzemeier U, Groth G, Butts C, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol.2004;15(1):19-27. doi: 10.1093/annonc/mdh031.
- Ricciardi GR, Russo A, Franchina T, et al. NSCLC and HER2: between lights and shadows. J Thorac Oncol. 2014;9(12):1750-1762. doi: 10.1097/JTO.0000000000000379.
- Gutierrez ME, Choi K, Lanman RB, et al. Genomic pro- filing of advanced non-small cell lung cancer in community settings: gaps and opportunities [published online April 13, 2017]. Clin Lung Cancer. doi: 10.1016/j.cllc.2017.04.004.
- Tan CS, Gilligan D, Pacey S. Treatment approaches for EG- FR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol. 2015;16(9):e447-e459. doi: 10.1016/S1470-2045(15)00246-6.
- Saad S, Huang K, Halmos B. Overcoming resistance to EGF receptor tyrosine kinase inhibitors in EGFR-mutated NSCLC. Lung Cancer Manage. 2014;3(6):459-476. doi: 10.2217/lmt.14.35.
- Mok TS, Wu Y-L, Ahn M-J, et al; AURA3 Investigators. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629-640. doi: 10.1056/NEJMoa1612674.
- Ramalingam S, Reungwetwattana T, Chewaskulyong B, et al. Osimertinib vs standard of care (SoC) EGFR-TKI as first- line therapy in patients (pts) with EGFRm advanced NSCLC: FLAURA. Ann Oncol. 2017;28(suppl 5);LBA2_PR. doi.org/10.1093/annonc/mdx440.050. academic.oup.com/an- nonc/article/4109962. Accessed Octber 27, 2017.
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039-1043. doi: 10.1126/ science.1141478.
- Turke AB, Zejnullahu K, Wu YL, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NS- CLC. Cancer Cell. 2010;17(1):77-88. doi: 10.1016/j.ccr.2009.11.022.
- Chung JH, Rho JK, Xu X, et al. Clinical and molecular evidences of epithelial to mesenchymal transition in acquired resistance to EGFR-TKIs. Lung Cancer. 2011;73(2):176-182.doi: 10.1016/j.lungcan.2010.11.011.
- Lee JK, Lee J, Kim S, et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol. 2017;35(26):3065-3074. doi: 10.1200/JCO.2016.71.9096.
- Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first-and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6(10):1118-1133. doi: 10.1158/2159-8290.CD-16-0596.
- Qiao H, Lovly CM. Cracking the code of resistance across multiple lines of ALK inhibitor therapy in lung cancer. Cancer Discov. 2016;6(10):1084-1086. doi: 10.1158/2159-8290.CD-16-0910.
- Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501.
- Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations asso- ciated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819-829. doi: 10.1056/NEJMoa1604958.
- Hellmann MD, Sanchez-Vega F, La K, et al. Molecular de- terminants of response and resistance to anti-PD-(L)1 blockade in patients with NSCLC profiled with targeted next-generation sequencing (NGS). Presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 9015.
- Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579-586. doi: 10.1200/JCO.2012.45.2011.
- Levy B, Hu ZI, Cordova KN, et al. Clinical utility of liquid diagnostic platforms in non-small cell lung cancer. Oncologist.2016;21(9):1121-1130. doi: 10.1634/theoncologist.2016-0082.
- Dagogo-Jack I, Bernicker E, Li T, et al. Genomic profiling of circulating tumor DNA (ctDNA) from patients (pts) with ad- vanced non-small cell lung cancer (NSCLC). Presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 9025.
- Sharma J, Shum E, Chau V, et al. The evolving role of biomarkers in personalized lung cancer therapy. Respiration. 2017;93(1):1-14. doi: 10.1159/000453086.
- Sacher AG, Paweletz C, Dahlberg SE, et al. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer [published correction appears in JAMA Oncol. 2016;2(8):1099]. JAMA Oncol. 2016;2(8):1014-1022. doi: 10.1001/jamaoncol.2016.0173.
- Khodakov D, Wang C, Zhang DY. Diagnostics based on nucleic acid sequence variant profiling: PCR, hybridization, and NGS approaches. Adv Drug Deliv Rev. 2016;105(Pt A):3-19.doi: 10.1016/j.addr.2016.04.005.
- Zheng D, Ye X, Zhang MZ, et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance. Sci Rep. 2016;6:20913. doi: 10.1038/srep20913.
- Oxnard GR, Thress KS, Alden RS, et al. Association be- tween plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(28):3375-3382. doi: 10.1200/JCO.2016.66.7162.
- Ihuegbu N, Banks KC, Fairclough SR, et al. Non-invasive detection of crizotinib resistance in ALK-rearranged lung adenocarcinoma directs treatment with next-generation ALK inhibitors. J Clin Oncol. 2016;24(suppl; abstr e20643).
- Chaudhuri A, Chabon JJ, Lovejoy AF, et al. Analysis of circulating tumor DNA in localized lung cancer for detection of molecular residual disease and personalization of adjuvantstrategies. Presented at: 2017 American Society of Clinical Oncol- ogy Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 8519. http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.8519.