Introduction
According to recent estimates, approximately 28,000 new cases of gastric cancer will be diagnosed in the United States in 2017.1 There has been a significant downward trend in the overall incidence of gastric cancer in recent years. The incidence per 100,000 people decreased from 12 in 1976 to 6.7 in 2013, and it is still declining.2 Gastric cancer mortality rates have also similarly decreased—the 5-year relative survival for all patients has doubled since the mid- 1970s. Unfortunately, despite these gains, about 70% of patients diagnosed with this disease will not be alive 5 years post diagnosis. Even for the subgroup of patients who present with localized disease without regional lymph node metastasis, the 5-year relative survival rate is an unsatisfactory 64%. Although great progress has been made in the management of gastric cancer, there is clear opportunity for continued improvement.
Worldwide, gastric cancer remains among the most commonly diagnosed malignancies, although the international annual incidence is also falling.3 The causes of gastric cancer remain multifactorial but perhaps the 2 strongest predisposing factors are infection with Helicobacter pylori and frequent ingestion of salted or smoked foods.4 While in Western nations the initial decrease in gastric cancer incidence began in the early to mid-20th century, a comparable trend has been noted only more recently in endemic areas such as Japan or South Korea.3 The widespread availability of food refrigeration and the successful treatment of active H. pylori infection have been identified as key interventions leading to the lower prevalence of gastric cancers. Nonetheless, although the worldwide per-capita rates of gastric cancer are decreasing, the overall number of new cases continues to grow with the increasing worldwide population and the median age at diagnosis continues to decrease.
In accordance with changes in domestic and worldwide incidence, shifts in histologic and distribution patterns have also occurred over the past several decades. The most common intestinal histologic subtype carries a better prognosis,5 but the diffuse histologic subtype, which carries a poor prognosis, typically affects younger patients and does not appear as dependent on environmental factors as does the intestinal histologic subtype. The diffuse histologic subtype now represents approximately 20% of gastric cancer diagnoses in recently reported American studies.6,7 Furthermore, a notable anatomic shift has occurred: Tumors of the gastric cardia have become more prevalent while the incidence of distal tumors has decreased.8 This trend parallels that seen in esophageal cancer, which may be a significant confluence due to the similar origin and behavior of gastric cardia tumors when compared with adenocarcinomas of the gastro- esophageal junction.9
Historical Management of Gastric Cancer and the Role of Radiotherapy
The sole proven curative intervention for gastric cancer is radical surgery, although there may be a role for endoscopic mucosal resection in patients with tumors limited to the lamina propria or muscularis mucosae without evidence of lymph node involvement.10 Radical resection of a gastric tumor that is limited to the submucosa can be curative; however, in patients with deeper tissue invasion or lymph node metastases, this procedure alone yields poor patient survival outcomes. Early randomized trials examined surgical techniques used in the management of gastric cancer in order to clarify the role of partial versus total gastrectomy. Multiple European studies demonstrated similar outcomes between partial and total gastrectomy for patients with distal tumors; however, total gastrectomy remains the standard of care for proximally located tumors.11,12
The role of extended lymphadenectomy in the treatment of gastric cancer remains controversial, despite a preponderance of data from large, randomized trials. Surgical lymph node levels are usually classified by the Japanese Gastric Cancer Association system and are used to determine the extent of lymphadenectomy needed. Briefly, removal of stations 1 to 6 (perigastric lymph nodes) is considered a D1 dissection, whereas removal of stations7-11 (celiac, common hepatic, and splenic lymph nodes) is considered a D2 dissection. More extensive lymphadenectomy, including removal of the para-aortic nodes, has been evaluated in the randomized setting, but it does not appear to confer a benefit over D2 dissection.13 Furthermore, the role of D2 resection, while accepted as standard in Japan, remains controversial in Europe and the United States. Although D1 dissection is associated with less operative morbidity and mortality than a D2 procedure, 15-year follow-up of patients in a Dutch randomized trial revealed a significant locoregional recurrence (LRR) benefit to carrying out the more extensive D2 surgery.14,15 Nonetheless, the high frequency of local failure and underwhelming patient survival rates observed in these trials suggest that surgery alone is unacceptable for all patients except those with early-stage disease.
An early trial from the British Stomach Cancer Group—which randomized patients to observation, adjuvant radiotherapy, or adjuvant chemotherapy following surgical resection—failed to demonstrate an overall survival (OS) benefit. Outcomes were generally discouraging; patient 5-year OS was only about 17% in any treatment group.16 However, there was a large reduction in LRR with the addition of adjuvant therapy to surgery. Radiotherapy in particular decreased the LRR from 27% to just 10%, suggesting that a more comprehensive treatment approach might yield better outcomes. An additional randomized trial carried out in China evaluated the role of neoadjuvant radiotherapy prior to radical resection for adenocarcinoma of the gastric cardia.17 In this study, patients either underwent surgery alone or received a preoperative dose of 40 gray (Gy) to the gastric cardia, gastroesophageal junction, and limited regional lymph nodes. A significant OS advantage (absolute risk reduction of approximately 10% at 5 years) was noted in the group receiving the neoadjuvant radiotherapy. The role of neoadjuvant radiation therapy, a strategy successfully applied to the management of other gastrointestinal cancers, is being further evaluated in ongoing clinical trials.
The Role of Radiotherapy in the Adjuvant Setting
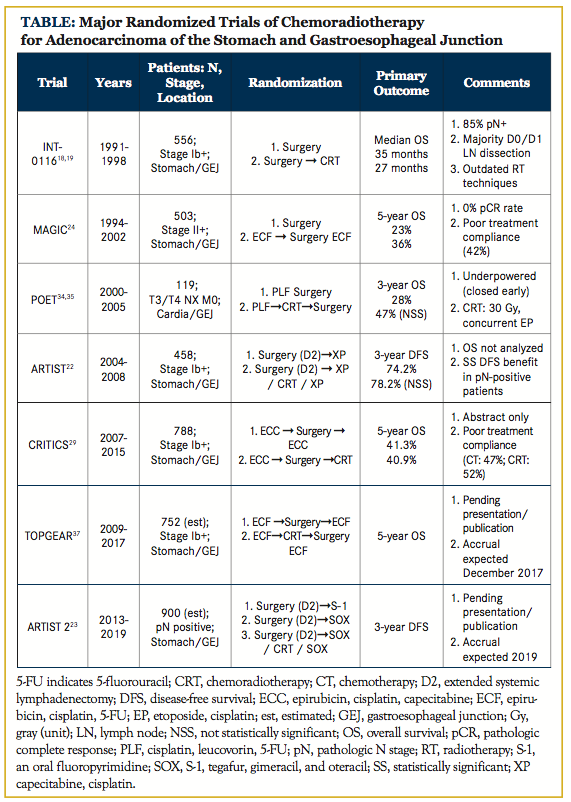
The benefits of adjuvant radiotherapy in the management of gastric cancer became more clearly defined in 2001 after the publication of the landmark Intergroup 0116 trial.18,19 Eligible patients had at least stage Ib adenocarcinoma of the stomach or gastroesophageal junction using the 3rd edition American Joint Committee on Cancer (AJCC) staging manual, although the majority of tumors were located in the distal stomach, were stage T3 or T4, and had associated nodal disease at diagnosis. Patients were randomized to undergo observation or adjuvant chemoradiotherapy following surgical resection. Chemotherapy consisted of 5 cycles of bolus 5-fluorouracil (5-FU) with leucovorin, and radiation therapy entailed delivery of a 45 Gy dose to the tumor bed and regional lymph nodes, primarily using opposed anterior and posterior fields concurrently with the second and third cycles of chemotherapy.
OS following adjuvant chemoradiotherapy was markedly improved: a median OS of 36 months was achieved in patients receiving adjuvant therapy compared with 27 months in those who underwent surgery alone. Additionally, the local failure rate (2% vs 8%) and regional failure rate (22% vs 39%) were better following adjuvant chemoradiotherapy. Distant metastatic disease rates were similar between the 2 arms: 16% following chemoradiotherapy plus surgery and 18% following surgery alone. These data confirm the benefit of adjuvant chemoradiotherapy in the postoperative setting, particularly in node-positive patients who receive no neoadjuvant therapy.
Despite these positive findings, several criticisms have been leveled against this trial. As expected, toxicity rates were significantly higher in patients undergoing chemoradiotherapy. Thirty-three percent of patients in the chemoradiotherapy arm suffered from grade 4 acute toxicity, and 4 treatment-related deaths were observed (secondary to cardiac toxicity, neutropenic sepsis, pulmonary fibrosis, and central line–associated fungemia). Although certainly concerning, these effects can likely be minimized by using modern chemotherapy delivery and radiotherapy techniques. Extrapolating from experience in rectal adenocarcinoma and nonrandomized gastric cancer studies, the use of either continuously infused 5-FU or oral capecitabine in lieu of bolus 5-FU is associated with less toxicity and likely achieves equivalent outcomes.20,21 Moreover, the delivery of radiotherapy has undergone several technological revolutions since this trial was carried out. Perhaps most significantly, highly conformal radiotherapy techniques, such as intensity-modulated radiotherapy (IMRT), have been introduced. A comprehensive discussion of advances in radiotherapy techniques and their applicability to gastric cancer is addressed later in this review.
The limited extent of lymph node dissection performed in most patients enrolled in the Intergroup 0116 trial has been a source of considerable criticism. Although a full D2 lymph node dissection was recommended by the investigators, only 10% of enrolled patients underwent this procedure. Furthermore, only 36% of patients underwent a D1 resection, while the remaining 54% of patients were treated with a D0 resection. Given the high rate of lymph node involvement, many have argued that chemoradiotherapy may have compensated for suboptimal lymph node dissection and may be unnecessary in patients who undergo more extensive surgery.
To address this shortcoming, the Korean randomized phase III ARTIST trial evaluated adjuvant chemoradiotherapy in patients with pathologic AJCC seventh edition stage IB-IIIC who had undergone R0 resection with full D2 lymphadenectomy.22 Previous studies carried out in Japan and Korea, the Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer (CLASSIC) and Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer (ACTS-GC) trials, respectively, demonstrated an OS benefit following the addition of adjuvant chemotherapy to more thorough surgical resection. In the investigational arm of the ARTIST trial, patients received 2 cycles of capecitabine and cisplatin together (XP) prior to chemoradiotherapy with capecitabine, followed by another 2 cycles of XP. The XP-only group was chosen as the control arm. The median follow-up was 5 years, after which time there were no significant differences in dis- ease-free survival (DFS) or OS. There was a trend toward improved DFS following the use of chemoradiotherapy (hazard ratio, 0.74; P = .09), which was the primary endpoint of the trial. Posthoc analyses revealed statistically significant DFS benefits for those patients with either node-positive disease or intestinal-type histology. A follow-up trial, the ARTIST 2, is currently accruing a similar patient cohort with positive lymph nodes, which is randomizing patients to either S-1 (an oral combination of tegafur, gimeracil, and oteracil), S-1 with oxaliplatin, or chemoradiotherapy. No results from this trial are currently available.23
The results discussed in this section suggest that postsurgical adjuvant chemoradiotherapy should be routinely used in patients who have undergone curative surgical resection in the absence of neoadjuvant therapy with additional risk factors such as pathologic invasion of the muscularis propria or positive lymph nodes, particularly in the setting of D1 or D0 lymphadenectomy.
Role of Chemoradiotherapy in the Era of Perioperative Chemotherapy
While the Intergroup 0116 trial established adjuvant chemoradiotherapy as the standard of care for resected gastric cancer in the United States, a few alternative treatment strategies have been employed. Foremost among these paradigms is perioperative chemotherapy, which was established following publication of the Medical Research Council’s MAGIC trial in 2006.24 Notably, this trial included tumors of the stomach, gastroesophageal junction, and esophagus, although the majority of tumors (74%) were located in the stomach. Patients who were randomized to perioperative chemotherapy were scheduled to receive 3 cycles of combination epirubicin, cisplatin, and 5-FU (ECF) prior to radical resection as well as 3 cycles of ECF in the postoperative adjuvant setting. Perioperative chemotherapy resulted in more primary tumor downstaging, and it increased both OS and progression-free survival. Additionally, patients who actually received neoadjuvant chemotherapy and underwent radical surgery were more likely to undergo R0 resection, although this finding did not achieve statistical significance on intention-to-treat analysis. However, treatment completion was challenging for most patients, as only 42% of those enrolled were able to complete the full chemotherapy schedule. Furthermore, no patient had a pathologic complete response (pCR) at the time of surgical resection, following 3 initial cycles of ECF.
Several other trials have examined the role of perioperative chemo- therapy with similar results, such as the French FNLCC/FFCD and EORTC 40954 trials.25,26 Although an OS benefit was not observed in the EORTC 40954 trial, which was closed early, secondary to poor accrual, both trials demonstrated improved R0 resection rates with neoadjuvant chemotherapy.
A meta-analysis comprising many of these trials confirmed the positive effect of neoadjuvant chemotherapy on OS, R0 resection rate, and primary tumor downstaging.27 As a result, there has been fierce debate over the past decade regarding whether adjuvant chemoradiotherapy or perioperative chemotherapy provides the best outcomes in patients with locally advanced gastric cancer.28 Although the ARTIST trial shed some light on this question, extrapolating these results is problematic for numerous reasons. Fortunately, the recently presented CRITICS trial, which is not yet available in manuscript form, should help guide treatment decisions.29 In this study, all patients received 3 cycles of neoadjuvant ECF or epirubicin, oxaliplatin, and 5-FU (EOF) prior to undergoing definitive surgical resection. Following surgery, patients were treated according to preoperative randomization, which consisted of an additional 3 cycles of ECF or EOF or chemoradiotherapy with concurrent XP. Extent of surgical resection was greater than that seen in the Intergroup 0116 study, with nearly 90% of patients receiving at least D1 lymphadenectomy and a median of 20 lymph nodes removed. The 5-year OS was approximately 41% in both arms, and although these results appear to compare favorably to both the MAGIC and Intergroup 0116 trials, there was no evidence of superiority for either arm. Grade 3 hematologic toxicity was slightly higher in the perioperative chemotherapy arm (44% vs 34%), but patients in both arms had difficulty completing protocol treatment (47% for perioperative chemotherapy, 52% for adjuvant chemoradiotherapy). In light of these findings, we do not recommend adjuvant chemoradiotherapy for patients who undergo R0 resection following neoadjuvant ECF unless they are unable to tolerate multiagent chemotherapy in the postoperative setting or are enrolled in a clinical trial.
The role of chemoradiotherapy is less well defined for patients who undergo surgical resection with either positive margins or gross residual disease, because no prospective data exist to guide treatment decisions in this setting. However, a retrospective review including patients from the Dutch lymphadenectomy trial revealed both an LRR benefit (6% vs 26%) and an OS benefit (66% vs 29%) at 2 years following the addition of chemoradiotherapy to R1 resection. A sub- sequent population-level analysis of the Netherlands Cancer Registry confirmed these findings,30,31 and a retrospective case series of patients who underwent adjuvant chemoradiotherapy noted equivalent OS and LRR in patients who underwent either R0 or R1 resection.32 Furthermore, in a randomized trial examining neoadjuvant chemotherapy for patients with esophageal cancer, long-term survival following R1 resection was achieved only in patients who received adjuvant chemoradiotherapy.33 Taken together, these data suggest that adjuvant chemoradiotherapy should be considered standard in patients with positive margins or gross residual disease, assuming that radiotherapy was not delivered preoperatively.
Role of Radiotherapy in the Neoadjuvant Setting
For many sites throughout the gastrointestinal tract, neoadjuvant or definitive chemoradiotherapy is gaining acceptance as an alternative to immediate surgical resection. In the United States, locally advanced rectal cancers and esophageal cancers are now routinely treated with neoadjuvant chemoradiotherapy following publication of the German Rectal and CROSS trials, respectively. Indeed, studies of esophageal cancer, which have typically included adenocarcinomas of the gastroesophageal junction and gastric cardia, may be particularly instructive when considering treatment for gastric malignancies. The POET trial randomized patients with Siewert Type I-III adenocarcinoma of the gastroesophageal junction to neoadjuvant chemotherapy or chemoradiotherapy. Neoadjuvant chemotherapy consisted of cisplatin, leucovorin, and 5-FU in combination, while neoadjuvant chemoradiotherapy included this regimen followed by radiotherapy administered with concurrent cisplatin and etoposide. Although this trial was limited by poor accrual and ultimately closed early, there was a strong trend toward improved OS with the addition of radiotherapy to neoadjuvant treatment. Additionally, the pCR rate and node positivity rate were improved with chemoradiotherapy despite a dose of only 30 Gy. Longer-term follow-up of 34 patients in this study, reported at the 2016 Annual Meeting of the American Society of Clinical Oncology, again noted an apparent OS advantage with chemoradiotherapy (39.5% vs 24.4% at 5 years), but these results failed to achieve statistical significance (P = .055).35 Neoadjuvant chemoradiotherapy confers several benefits relative to the postoperative setting, including smaller target volumes, improved patient compliance, and removal of the irradiated normal tissue at the time of resection, which may limit late-onset toxicity. The use of neoadjuvant, rather than adjuvant, chemoradiotherapy may not only be better tolerated by patients, but also be more oncologically efficacious. Retrospective data from The University of Texas MD Anderson Cancer Center have demonstrated the tolerability of this approach in patients with a minimum of T2N0 gastric cancer, and patients in this study, reported at the 2016 Annual Meeting of the American Society of Clinical Oncology, again noted an apparent OS advantage with chemoradiotherapy (39.5% vs 24.4% at 5 years), but these results failed to achieve statistical significance (P = .055).35
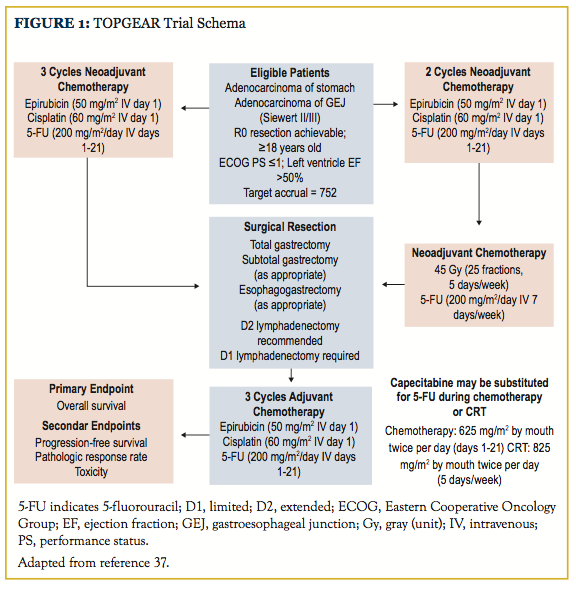
Neoadjuvant chemoradiotherapy confers several benefits relative to the postoperative setting, including smaller target volumes, improved patient compliance, and removal of the irradiated normal tissue at the time of resection, which may limit late-onset toxicity. The use of neoadjuvant, rather than adjuvant, chemoradiotherapy may not only be better tolerated by patients, but also be more oncologically efficacious. Retrospective data from The University of Texas MD Anderson Cancer Center have demonstrated the tolerability of this approach in patients with a minimum of T2N0 gastric cancer, and 80% of these patients ultimately underwent R0 resection with a 20% pCR rate.36 Given this apparent benefit, the randomized phase III TOPGEAR study, which is expected to complete patient accrual in December 2019, was designed with the hope of elucidating the role of neoadjuvant chemoradiotherapy.37 The investigational arm of this trial employs perioperative ECF as prescribed in the MAGIC trial, except chemoradiotherapy is substituted for the third neoadjuvant cycle of chemotherapy (Figure 1). Chemoradiotherapy, delivered con- currently with either continuously infused 5-FU or oral capecitabine, consists of a 45 Gy dose to the entire stomach, any perigastric tumor extension, and regional lymph nodes. A recently published interim analysis suggests similar rates of surgical complications and treatment compliance in the investigational and control arms of this trial, but oncologic outcomes are not yet available.38 Final results from the TOPGEAR study should help clarify the role of radiotherapy in the setting of perioperative chemotherapy. A summary of several major randomized trials is available in the Table.
Role of Radiotherapy in Cases of Unresectable Gastric Cancer
In contrast to data regarding patients with resectable gastric cancer, there are limited data to guide the treatment of patients with nonmetastatic, unresectable gastric cancers. Nonetheless, the available literature suggests that chemoradiotherapy may have a role in achieving durable palliation and conversion to resectable disease. In the midtwentieth century, randomized data demonstrated a clear survival benefit for patients with unresectable cancers of the stomach when 5-FU was added to palliative radiotherapy, although no patients were reported to have received an attempted curative resection.39 More recently, a Japanese phase II study that employed chemoradiotherapy for patients with unresectable locally advanced gastric cancer demonstrated an eventual resection rate of 33.3% and an overall pCR rate of 13.3%.40 In this study, 40 Gy in 2 Gy daily fractions were delivered to the primary tumor and regional lymph nodes with concurrent S-1 and cisplatin. The authors also reported that all 30 patients required hospitalization due to disease-related symptoms at the time of diagnosis; however, 97% were discharged after 1 cycle of chemo- therapy, suggesting that even patients who did not reach surgery benefited from treatment.
An alternative approach to treatment of unresectable gastric cancer is multi-agent chemotherapy alone. Although a thorough discussion of this approach is beyond the scope of this review, many regimens can be used in this setting. However, in patients with good performance status and minimal comorbidity, we believe that incorporation of radiotherapy into gastric cancer treatment regimens could provide the highest likelihood of conversion to oncologic resectability and long-term disease control. Consequently, we recommend that such patients be evaluated in the multidisciplinary setting with appropriate input from surgeons, medical oncologists, and radiation oncologists with extensive experience in the treatment of gastric malignancies.
Radiotherapy Planning and Delivery
The Intergroup 0116 trial, which set the standard of care for adjuvant chemoradiotherapy, employed radiation techniques that are considered outdated in the modern radiation oncology clinic. Since the time of this trial, 3-dimensional conformal radiotherapy, image-guided radiotherapy (IGRT), IMRT, and volumetric modulated arc therapy (VMAT) have become commonplace in the United States. These techniques can achieve extensive normal tissue sparing with excellent target coverage (Figure 2). Without question, a minimum standard in the definitive setting should be CT-based simulation with 3-dimensional planning, given the large volumes typically employed and multiple radiosensitive organs at risk in near proximity to the tar- get. Target delineation in gastric cancer is extremely complicated: even with the simple beam arrangements utilized in the Intergroup 0116 trial, approximately one-third of plans submitted for central review were in violation of the prescribed protocol.41 With the introduction of more conformal techniques and tighter margins, consideration and knowledge of anatomical patterns of spread is crucial.
Prior to initiation of radiotherapy, appropriate imaging and work- up are crucial to guide the treatment planning process. All patients with gastric cancer should undergo esophagogastroduodenoscopy and biopsy of the primary tumor, as well as endoscopic ultrasound to determine depth of invasion and to assess regional lymph nodes. A CT scan of the chest, abdomen, and pelvis, with both oral and intravenous contrast, is critical to assess regional lymphadenopathy and rule out metastatic disease. Although PET is not as sensitive in detection of lymph node and distant metastases secondary to limited 18F-deoxyglucose avidity in certain histologic subtypes, the use of combined PET/CT is now recommended by the National Comprehensive Cancer Network42 and may be useful for radiotherapy target delineation. A full discussion of laparoscopic staging with peritoneal cytology is beyond the scope of this review, but its use may be appropriate in patients for whom neoadjuvant therapy is planned.
Before CT simulation and each radiotherapy fraction, patients should fast for several hours in order to maximize reproducibility of gastric filling. Patients are typically positioned supine, with arms immobilized above the head to allow multifield or volumetric modulated arc therapy plans. The use of a custom immobilization device is recommended to minimize set-up uncertainties, and intravenous contrast is essential for the proper delineation of lymphatic target volumes. We recommend obtaining the simulation CT with and without oral contrast for optimal treatment planning, as well as contouring of the primary tumor or resection bed. Motion management strategies, which may include 4-D CT, respiratory gating, or abdominal compression, should be considered because target volumes are susceptible to substantial respiratory movement. Finally, for patients who have undergone surgical resection, fusion of available preoperative imaging is essential, as is thorough review of the operative note and surgical pathology. Target delineation in both the neoadjuvant and adjuvant setting is complicated, requiring a detailed understanding of regional lymphatic spread patterns and postoperative anatomy. Furthermore, these volumes may vary significantly depending on the location of the primary tumor and extent of surgical resection, if performed.
In the majority of patients, target volumes will include the primary tumor or resection bed, gastric remnant (if present), and regional lymph nodes; however, in some patients, inclusion of the duodenal stump or surgical anastomosis may be advisable.41 Given the results of the Intergroup 0116 trial, 45 Gy given in 25 daily fractions is considered standard, but a boost of 5.4 to 9 Gy may be given for positive margins, gross residual disease, or definitive treatments. An excellent contouring atlas, published by Wo and colleagues in 2013, is available and highly useful for target delineation,43 as is an additional guide that is specifically tailored to patients treated with D2 lymphadenectomy.44 Organs at risk, including the kidneys, liver, small bowel, lungs, heart, and spinal cord, should be contoured and appropriately constrained. Given the high anatomic variability of this region, we recommend an IGRT technique if highly conformal methods such as IMRT are employed.
Future Directions and Conclusions
Although substantial improvements in the management of gastric cancer have been made over the past several decades, overall out- comes remain disappointing with unsatisfactory cure rates in all but the earliest-stage patients. The optimal treatment paradigm for most patients with gastric cancer remains unclear and may vary with tumor histology and location. Furthermore, it appears that the traditional pillars of oncology are approaching their limits, and that future innovations are sorely needed. Targeted agents, novel radiosensitizers, and even new modalities may be necessary to improve upon the successes of the past half-century. However, radiotherapy continues to play a crucial role for many patients, particularly those who did preoperative therapy, are found to have positive lymph nodes, retain residual disease following surgery, or are unresectable at diagnosis. The role of a trimodality approach in the neoadjuvant setting is promising, but its use is still investigational.
Acknowledgments
We would like to thank Marion Hartley, PhD, science writer for clinical research at the Ruesch Center for the Cure of Gastrointestinal Cancers, Georgetown University, for her edits during the composition of this manuscript.
Affiliations: Michael C. Repka and Keith R. Unger are with the Department of Radiation Medicine, and Mohamed E. Salem is with the Department of Oncology, all at Georgetown University Hospital, Washington, DC.
Send correspondence to: Keith R. Unger, MD, 3800 Reservoir Rd NW, Department of Radiation Medicine, Lower Level Bles Bldg, Georgetown University Hospital, Washington, DC 20007. E-mail: [email protected].
Conflicts of interest and financial disclosures: None. Funding disclosures: None.

References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387.
- Howlander N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. National Cancer Institute website. http:// seer.cancer.gov/csr/1975_2013/. Published April 2016. Updated September 12, 2016. Accessed March 14, 2017.
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16-27. doi: 10.1158/1055-9965.EPI-15-0578.
- Parkin DM. The global health burden of infection-associated can- cers in the year 2002. Int J Cancer. 2006;118(12):3030-3044.
- Petrelli F, Berenato R, Turati L, et al. Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: a systematic review and meta-analysis. J Gastrointest Oncol. 2017;8(1):148-163. doi: 10.21037/jgo.2017.01.10.
- Kunz PL, Gubens M, Fisher GA, et al. Long-term survivors of gastric cancer: a California population-based study. J Clin Oncol. 2012;30(28):3507-3515.
- Raigani S, Hardacre JM, Kim J, Ammori JB. Trends in the surgical treatment of gastric adenocarcinoma. Ann Surg Oncol. 2014;21(2):569- 574. doi: 10.1245/s10434-013-3314-x.
- Camargo MC, Anderson WF, King JB, et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60(12):1644-1649. doi: 10.1136/gut.2010.236737.
- Kalish RJ, Clancy PE, Orringer MB, Appelman HD. Clinical, epidemiologic, and morphologic comparison between adenocarcinomas arising in Barrett’s esophageal mucosa and in the gastric cardia. Gastroenterology. 1984;86(3):461-467.
- Choi KS, Jung HY, Choi KD, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gas- trointest Endosc. 2011;73(5):942-948. doi: 10.1016/j.gie.2010.12.032.
- Gouzi JL, Huguier M, Fagniez PL, et al. Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. a French prospective controlled study. Ann Surg. 1989;209(2):162-166.
- Bozzetti F, Marubini E, Bonfanti G, et al. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg. 1999;230(2):170-178.
- Sasako M, Sano T, Yamamoto S, et al; Japan Clinical Oncology Group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359(5):453-462. doi: 10.1056/NEJMoa0707035.
- Bonenkamp JJ, Hermans J, Sasako M, et al; Dutch Gastric Cancer Group. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340(12):908-914.
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11(5):439-449. doi: 10.1016/ S1470-2045(10)70070-X.
- Hallissey MT, Dunn JA, Ward LC, Allum WH. The second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: five-year follow-up. Lancet. 1994;343(8909):1309-1312.
- Zhang ZX, Gu XZ, Yin WB, et al. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC)--report on 370 patients. Int J Radiat Oncol Biol Phys. 1998;42(5):929-934.
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725-730.
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30(19):2327-2333. doi: 10.1200/ JCO.2011.36.7136.
- Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst. 2015;107(11). doi: 10.1093/jnci/djv248.
- Lee HS, Choi Y, Hur WJ, et al. Pilot study of postoperative adjuvant chemoradiation for advanced gastric cancer: adjuvant 5-FU/ cisplatin and chemoradiation with capecitabine. World J Gastroenterol. 2006;12(4):603-607.
- Park SH, Sohn TS, Lee J, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, including survival and subset analyses. J Clin Oncol. 2015;33(28):3130-3136. doi: 10.1200/ JCO.2014.58.3930.
- Park SH, Lee SJ, Kim ST, et al. Multicenter phase III trial of adjuvant chemoradiotherapy in stomach tumors 2 (ARTIST 2). Paper presented at: 2015 Gastrointestinal Cancers Symposium. J Clin Oncol. 2015;33(suppl 3; abstr TPS228).
- Cunningham D, Allum WH, Stenning SP, et al; MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11-20.
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adeno- carcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715-1721. doi: 10.1200/JCO.2010.33.0597.
- Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28(35):5210-5218. doi: 10.1200/JCO.2009.26.6114.
- Xiong B-H, Cheng Y, Ma L, Zhang CQ. An updated meta-analysis of randomized controlled trial assessing the effect of neoadjuvant chemotherapy in advanced gastric cancer. Cancer Invest. 2014;32(6):272- 284. doi: 10.3109/07357907.2014.911877.
- Macdonald JS. Gastric cancer--new therapeutic options. N Engl J Med. 2006;355(1):76-77.
- Verheij M, Jansen EPM, Cats A, et al. A multicenter randomized phase III trial of neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy in resectable gastric cancer: first results from the CRITICS study. Paper presented at: 2016 ASCO Annual Meeting. J Clin Oncol. 2016;34(suppl; abstr 4000).
- Dikken JL, Jansen EPM, Cats A, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol. 2010;28(14):2430-2436. doi: 10.1200/ JCO.2009.26.9654.
- Stiekema J, Trip AK, Jansen EP, et al. Does adjuvant chemoradiotherapy improve the prognosis of gastric cancer after an r1 resection? results from a Dutch cohort study. Ann Surg Oncol. 2015;22(2):581- 588. doi: 10.1245/s10434-014-4032-8.
- Stiekema J, Trip AK, Jansen EP, et al. The prognostic significance of an R1 resection in gastric cancer patients treated with adjuvant chemoradiotherapy. Ann Surg Oncol. 2014;21(4):1107-1114. doi: 10.1245/s10434-013-3397-4.
- Kelsen DP, Winter KA, Gunderson LL, et al; Radiation Therapy Oncology Group; USA Intergroup. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25(24):3719-3725.
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27(6):851-856. doi: 10.1200/ JCO.2008.17.0506.
- Stahl M, Riera-Knorrenschild J, Stuschke M, et al. Preoperative chemoradiotherapy and the long-term run in curative treatment of locally advanced oesophagogastric junction adenocarcinoma: update of the POET phase III study. Paper presented at: 2016 ASCO Annual Meeting. J Clin Oncol. 2016;34 (suppl; abstr 4031).
- Chakravarty T, Crane CH, Ajani JA, et al. Intensity-modulated radiation therapy with concurrent chemotherapy as preoperative treatment for localized gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 2012;83(2):581-586. doi: 10.1016/j.ijrobp.2011.07.035.
- Leong T, Smithers BM, Michael M, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer. 2015;15:532. doi: 10.1186/s12885-015-1529-x.
- Leong T, Smithers BM, Haustermans K, et al. TOPGEAR: A randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: interim results from an international, intergroup trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol. 2017. doi: 10.1245/ s10434-017-5830-6. [Epub ahead of print.]
- Moertel CG, Childs DS Jr, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet. 1969;2(7626):865-867.
- Saikawa Y, Kubota T, Kumagai K, et al. Phase II study of chemo- radiotherapy with S-1 and low-dose cisplatin for inoperable advanced gastric cancer. Int J Radiat Oncol Biol Phys. 2008;71(1):173-179.
- Smalley SR, Gunderson L, Tepper J, et al. Gastric surgical adjuvant radiotherapy consensus report: rationale and treatment implementation. Int J Radiat Oncol Biol Phys. 2002;52(2):283-293.
- Gastric cancer--NCCN evidence blocks. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/ physician_gls/pdf/gastric_blocks.pdf. Updated March 2016. Accessed March 21, 2017.
- Wo JY, Yoon SS, Guimaraes AR, et al. Gastric lymph node contouring atlas: a tool to aid in clinical target volume definition in 3-dimensional treatment planning for gastric cancer. Pract Radiat Oncol. 2013;3(1):e11-e19. doi: 10.1016/j.prro.2012.03.007.
- Yoon HI, Chang JS, Lim JS, et al. Defining the target volume for post-operative radiotherapy after D2 dissection in gastric cancer by CT-based vessel-guided delineation. Radiother Oncol. 2013;108(1):72- 77. doi: 10.1016/j.radonc.2013.05.025.