Introduction
Breast cancer is a heterogeneous disease, with approximately 20% of breast cancers exhibiting amplification of the HER2 gene.1 This gene was discovered more than 30 years ago and was recognized to confer a poor prognosis.2-4 The approval of trastuzumab for the treatment of HER2-positive metastatic breast cancer in 1998 represented a major breakthrough for these patients. It took 8 more years to receive FDA approval in the adjuvant setting. Since then, trials have been conducted to identify the appropriate duration of therapy in combination with chemotherapy. More recently, trials incorporating newer HER2-targeted therapies, either given as dual blockade or sequentially following standard trastuzumab adjuvant therapy, have been reported. Here, we review the current data on HER2 blockade in the adjuvant therapy of breast cancer.Randomized Adjuvant Trastuzumab Trials
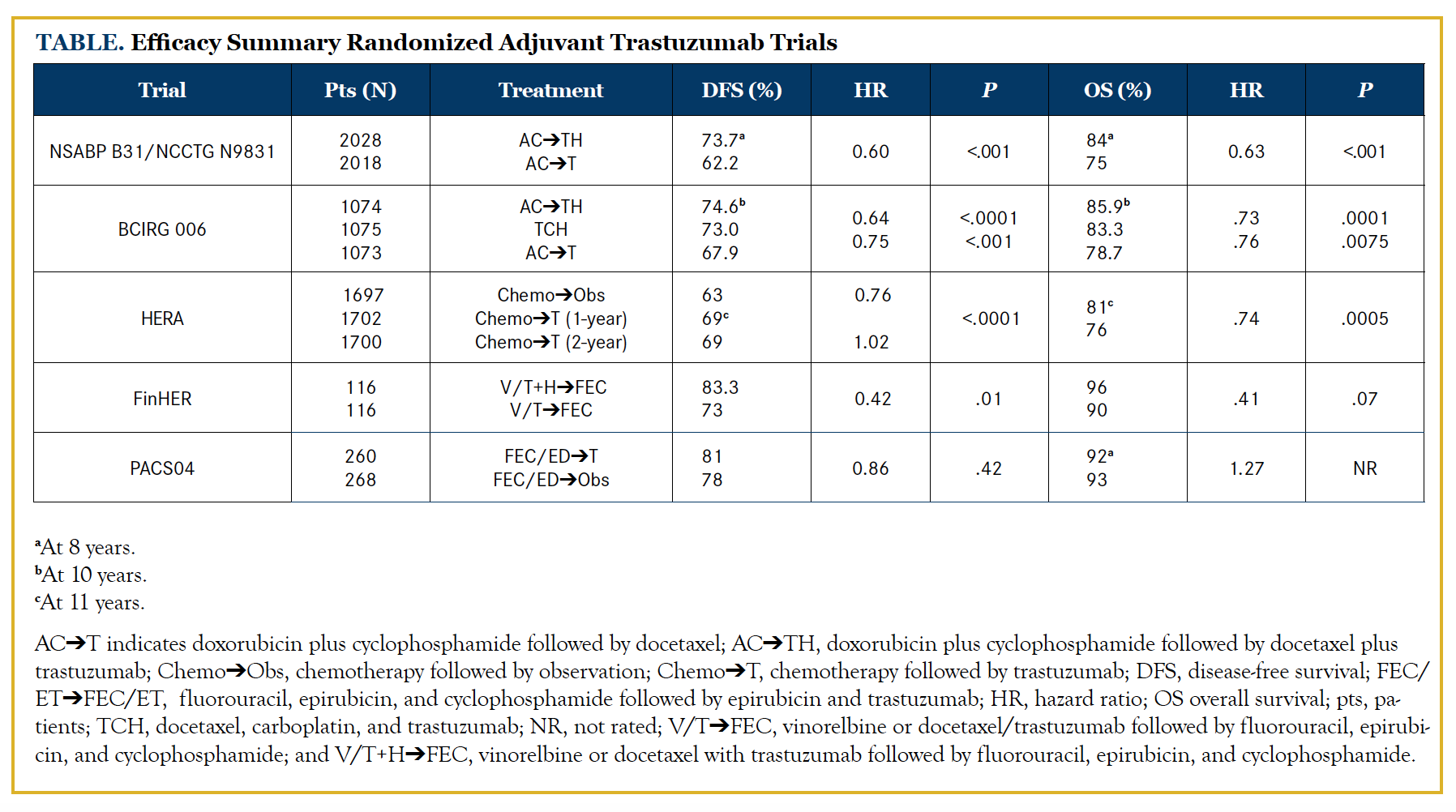
Several large, randomized adjuvant trastuzumab clinical trials (Table) have shown that the addition of 1 year of trastuzumab improves not only disease-free survival (DFS) but also overall survival (OS).5-7 In the FINHer study, trastuzumab was administered for 9 weeks, not 1 year, but still showed that the addition of trastuzumab improved DFS. Two smaller trials showed a nonstatistical significant improvement in DFS.8,9 O’Sullivan and colleagues conducted a meta-analysis of data from HERA, NCCTG N9831, NSABP-B31, PACS04, and the FinHER trials, focusing on patients with small (≤2 cm) HER2-positive breast cancers. After a median follow-up of 8 years of 2263 patients with HER2-positive and hormone receptor (HR)–positive breast cancer, the addition of trastuzumab improved DFS (75.7% to 82.7%) and OS (88.4% to 92.2%). In the 1957 patients with HR-negative disease, there was also improvement in DFS (66.6% to 76%) and OS (78.8% to 87.6%).10 Patients with small HER2-positive tumors may also have a significant risk of relapse.11 The MD Anderson series reviewed 965 T1aN0M0 breast cancers diagnosed over a 12-year period. Patients who received adjuvant chemotherapy or trastuzumab were excluded. Ten percent of patients had HER2-positive tumors. At a median follow-up of 74 months, the 5-year relapse-free survival (RFS) rates were 77.1% and 93.7% in patients with HER2-positive and HER2-negative tumors, respectively (P <.001).11 In contrast, Vaz-Luis and colleagues carried out a prospective cohort study from the National Comprehensive Cancer Network’s Cancer Outcomes Database in patients with T1a and T1b, node-negative, HR-negative, HER2-positive breast cancer. Their study showed that patients who did not receive trastuzumab or chemotherapy had a much higher 5-year distant relapse-free survival of 93% and 94%, respectively.12NSABP-B31 and NCCTG N9831 Trials
Perez and colleagues published the joint analysis of these 2 studies, which included 4046 patients. NSABP-B31 compared doxorubicin (A) and cyclophosphamide (C) followed by paclitaxel (T) every 3 weeks with the same regimen plus trastuzumab (H) for 52 weeks initiated concomitantly with paclitaxel. NCCTG N9831 compared 3 regimens: AC followed by weekly paclitaxel (group A), the same regimen followed by 52 weeks of trastuzumab (group B), and the same regimen plus 52 weeks of trastuzumab initiated concomitantly with paclitaxel (group C). Group B was excluded from the joint analysis of these 2 trials. The median time on study was 8.4 years. The addition of trastuzumab to chemotherapy improved the 10-year OS from 75.2% to 84% and the 10-year DFS from 62.2% to 73.7%.6
BCIRG 006
This trial randomized 3222 patients with HER2-positive breast cancer with node-positive or high-risk node-negative disease to standard AC followed by docetaxel (ACT), or AC followed by docetaxel with trastuzumab (ACTH) for 1 year, or the combination of docetaxel with carboplatin and trastuzumab (TCH) for 1 year. This trial was unique in that it included a nonanthracycline arm. After 10.3 years of follow-up, the 2 trastuzumab arms showed a statistically significant improvement in DFS and OS: DFS rates were 74.6% with ACTH, 73% with TCH, and 67.9% with ACT, while OS rates were 85.9%, 83.3%, and 78.7%, respectively.7
Smaller Tumors in Node-Negative Patients APT Tolaney et al conducted a single-arm, nonrandomized trial to evaluate if 12 weeks of adjuvant paclitaxel in combination with trastuzumab was effective in patients with node-negative, small (<3 cm), HER2-positive breast cancer.16 They enrolled 410 patients and reported a 7-year DFS of 93.3%.17 Of note, only 36 patients (9%) had tumors between 2 cm and 3 cm, while the majority of patients (more than 70%) had T1b or T1c tumors. Given the improvements in pathologic complete response noted with the incorporation of pertuzumab in the neoadjuvant setting for tumors over 2 cm, these data should not be broadly applied for all patients with tumors less than 3 cm. Also important to note is that two-thirds of patients had HR-positive tumors, and recurrences in this subset may be seen well after 3 years.
HERA
This international, randomized, phase III trial of 5102 women with HER2-positive breast cancer compared 1 or 2 years of trastuzumab with observation alone in patients who completed at least 4 cycles of neoadjuvant or adjuvant chemotherapy (1697 in observation, 1702 in 1-year trastuzumab, and 1700 in 2-year trastuzumab group). Three patients had no evidence of having provided written informed consent to participate.
After a median follow-up of 11 years, the patients who received 1 year of trastuzumab had a statistically significant improvement in 10-year DFS when compared with the patients who did not receive trastuzumab (69% vs 63%). Despite the effect of selective crossover, the hazard ratio for OS at 11 years of median follow-up was 0.74 (95% CI, 0.64-0.86). The absolute benefit in 12-year OS was 6.5%. Importantly, there was no advantage of a 2-year duration of therapy.5
FinHER
In this open-label, phase III, prospective multicenter trial, 1010 women with axillary node-positive or high-risk node-negative breast cancer were randomized to receive 3 cycles of docetaxel or vinorelbine, followed in both groups by 3 cycles of fluorouracil (F), epirubicin (E), and cyclophosphamide (FEC). Patients with HER2-positive breast cancer (n = 232) were further treated with trastuzumab or no additional therapy. Even with the shorter course of trastuzumab after a median follow-up of 8 years, distant DFS (83.3% vs 73%) and OS (91.3% vs 82.3%) favored the trastuzumab arm, but the difference in OS was not statistically significant.8
PACS04
In this study, 3010 patients with operable node-positive breast cancer were randomly assigned to receive adjuvant anthracycline-based chemotherapy with or without docetaxel. For HER2-positive patients (n = 528), there was a second randomization to sequential 1 year of trastuzumab or observation. The 3-year DFS was 78% versus 81% favoring the trastuzumab arm. There was no significant difference in OS (95% trastuzumab vs 96% observation).9 Possible hypotheses to explain these findings include the small sample size and perhaps the sequential use of trastuzumab as opposed to concomitant administration.
De-escalation of Therapy
HERA and PHARETwo of the more mature trials have evaluated the lengths of trastuzumab treatment and have failed to show that either shorter or longer duration is better than the established 1 year of trastuzumab. More recently, in results of a study presented at the 2017 American Society of Clinical Oncology Annual Meeting, a shorter course of adjuvant trastuzumab was reported to reduce cardiac toxicity.
As summarized above, HERA did not show any benefit to a 2-year duration of therapy.5 In PHARE, an open-label randomized phase III trial, 3384 patients with HER2-positive early breast cancer who received at least 4 cycles of chemotherapy and had received up to 6 months of adjuvant trastuzumab were randomized to continue trastuzumab for another 6 months or to stop trastuzumab at 6 months. This was a noninferiority trial designed to detect a 2% difference in recurrence. After 3.5 years of follow-up, the study failed to meet the prospectively established noninferiority endpoint, set at a hazard ratio of 1.15.13 Despite the statistically significant increased rate of cardiac events that occurred in patients assigned to 12 months of treatment (5.7% vs 1.9%), results from this trial indicated that 1 year of adjuvant trastuzumab should remain the standard of care.
Short-HER
In this study, patients were randomized to receive 1 year of trastuzumab plus chemotherapy (“long group”) or 9 weeks of trastuzumab plus chemotherapy (“short group”). The primary endpoints were DFS and OS. Secondary endpoints included failure rate at 2 years and the incidence of cardiac events. The 5-year DFS did not achieve noninferiority in the group as a whole, but in an analysis comparing DFS in patients with earlier-stage disease (stage I and II) to locally advanced disease (stage III), there was a suggestion that short was not inferior to longer treatment. There was no difference in OS at 5 years. Notably, there was an ongoing decline in left ventricular ejection fraction (LVEF) and significantly more cardiac events for the long group. In contrast, there was a much slower decline in LVEF for patients in the short group over 18 months, and fewer cardiac events. It was concluded that patientswith more advanced disease appear to derive greater benefit from a longer duration of trastuzumab, and that for a select group of patients who cannot tolerate 12 months of therapy, a shorter duration may be reasonable.14 Jones and colleagues conducted a single-group, open-label phase II study to evaluate if a nonanthracycline-containing regimen can be an option for the adjuvant treatment of women with lower-risk (stage I to II) HER2-positive breast cancer. There were 493 patients enrolled who received four 21-day cycles of docetaxel plus cyclophosphamide plus trastuzumab followed by 1 year of trastuzumab. Results demonstrated a 3-year DFS of 96.9% and 3-year OS of 98.7%. The authors concluded that a short (4-cycle), nonanthracycline-containing regimen was effective.15 While Slamon and colleagues had already established the TCH regimen with carboplatin in the BCIRG-006 trial summarized above, these study results provide good evidence to consider cyclophosphamide as an option in patients who are not considered fit to receive carboplatin.
Smaller Tumors in Node-Negative Patients
APTTolaney et al conducted a single-arm, nonrandomized trial to evaluate if 12 weeks of adjuvant paclitaxel in combination with trastuzumab was effective in patients with node-negative, small (<3 cm), HER2-positive breast cancer.16 They enrolled 410 patients and reported a 7-year DFS of 93.3%.17 Of note, only 36 patients (9%) had tumors between 2 cm and 3 cm, while the majority of patients (more than 70%) had T1b or T1c tumors. Given the improvements in pathologic complete response noted with the incorporation of pertuzumab in the neoadjuvant setting for tumors over 2 cm, these data should not be broadly applied for all patients with tumors less than 3 cm. Also important to note is that two-thirds of patients had HR-positive tumors, and recurrences in this subset may be seen well after 3 years.
Additional Targeted Therapy Beyond Trastuzumab
Although outcomes have significantly improved with the incorporation of trastuzumab in the adjuvant setting for 1 year, relapses still occur. As such, researchers have sought to identify a benefit for the receipt of additional targeted therapy either in combination with trastuzumab, or after completion of 1 year of therapy. Agents that have been investigated include: lapatinib, a small-molecular TKI of both EGFR and HER2; neratinib, an irreversible pan-inhibitor of EGFR, HER2, and HER4; and bevacizumab, an antiangiogenic agent.Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization (ALTTO)
Given the improvements noted in the neoadjuvant setting with combination lapatinib and trastuzumab therapy in the NeoALTTO trial, there was enthusiasm to evaluate this combination in the adjuvant setting as well. The ALTTO trial was a phase III randomized trial that included 8381 patients randomized to 1 of 4 groups: control group (trastuzumab alone [T]); lapatinib-alone group ([L], closed early); combination group (L+T); and a sequential arm (T➔L). The results were disappointing: After 5-year follow-up treatment with lapatinib plus trastuzumab, either sequentially or concurrently, 6-year DFS rates were 85% versus 82% (L+Tvs T), and 84% versus 82% (T➔L vs T). The 6-year OS rates were 93%, 92%, and 91% for L+T, T ➔L, and T, respectively.18
BETH
This randomized, phase III, open-label study evaluated the addition of bevacizumab to anthracycline and nonanthracycline (TCH) adjuvant trastuzumab regimens. In both groups, patients continued trastuzumab with or without bevacizumab after chemotherapy to complete 1 year of targeted therapy. A total of 3509 patients were enrolled with either node-positive or high-risk node-negative disease, with the latter group accounting for 41% of the population. At a median follow-up of 38 months, the invasive DFS (iDFS) rates were 92% for both TCH groups. A secondary endpoint compared iDFS in the anthracycline versus TCH groups, and also found no significant difference between the bevacizumab and no-bevacizumab regimens.19 It is important to note that this trial was not designed to compare anthracyclineversus nonanthracycline-based therapy, but to answer the question about any potential benefit of the addition of bevacizumab. Given the additional toxicity, as expected, in the bevacizumab arms, without any added benefit, this strategy has not been further explored.
ExteNET
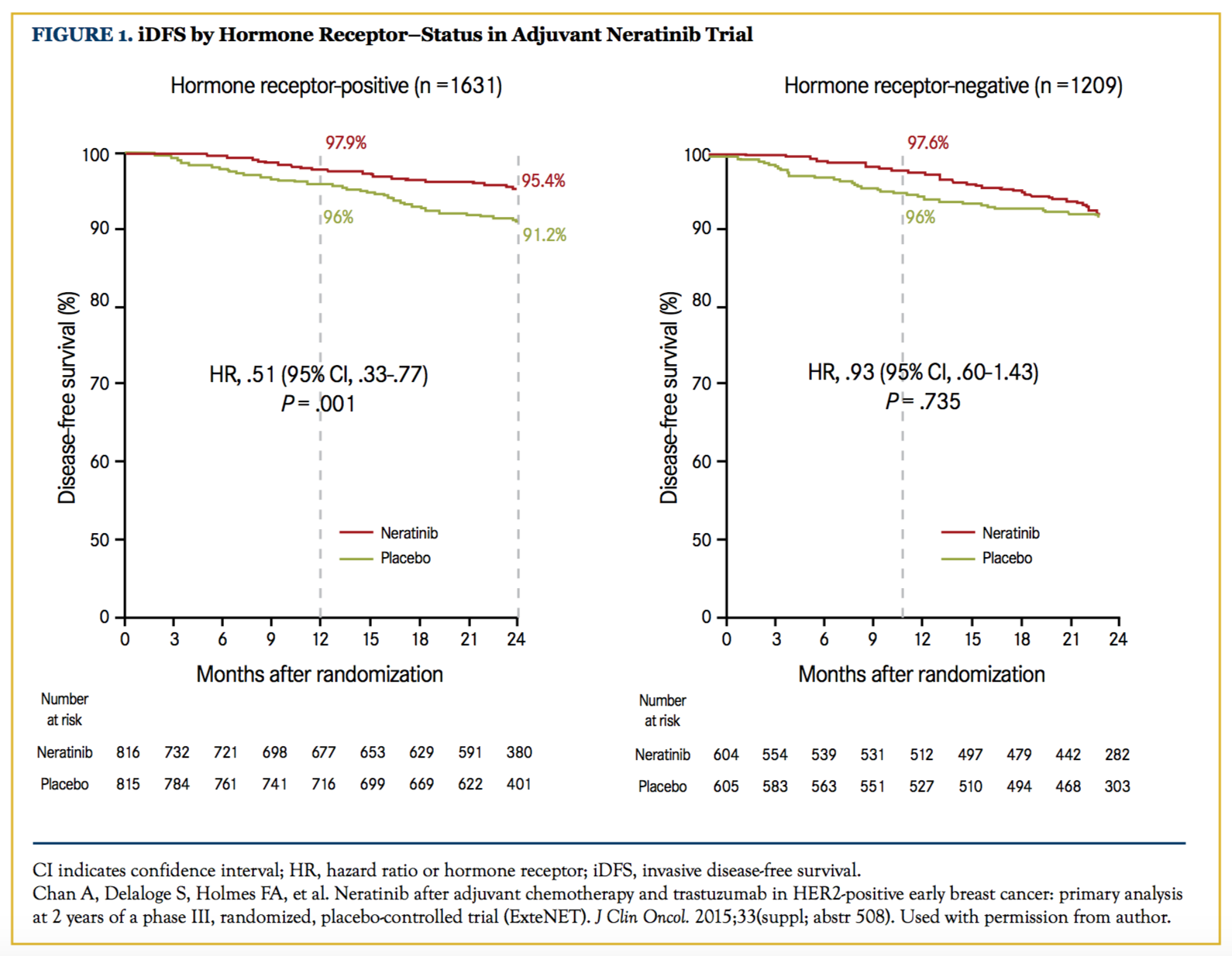
This study tested neratinib, an oral anti-HER2 TKI, after adjuvant chemotherapy and trastuzumab in HER2-positive early breast cancer.20 This randomized, phase III, placebo-controlled trial enrolled 2840 patients with HER2-positive breast cancer, stage I to III, who had completed adjuvant trastuzumab for 1 year; they were then randomized to 1 additional year of neratinib or placebo. The 2-year iDFS was 93.9% in the neratinib arm versus 91.6% in the placebo arm.21 The updated 3-year iDFS was 92% with neratinib versus 89.9% with placebo.22 The results were recently updated in the 2017 Oncologic Drugs Advisory Committee (ODAC) meeting, and the 5-year iDFS was 90.2% with neratinib versus 87.7% with placebo.23 Interestingly, when the results were stratified by receptor status, the benefit was confined to the HR-positive patients (hazard ratio of 0.51 vs 0.93)21 (Figure 1). The authors have hypothesized that the mechanism of action of neratinib may be beneficial in modulating estrogen receptor sensitivity to hormonal therapy, although further studies are required to evaluate this possibility. The incidence of grade 3 diarrhea was nearly 40% (no study-mandated prophylactic loperamide was required). Subsequent studies with prophylactic strategies have shown substantial improvement in this complication. The ODAC committee voted to recommend approval of neratinib for the extended adjuvant treatment of HER2-positive early-stage breast cancer, based on the finding that the risk-benefit profile of neratinib is favorable. Subsequently, the FDA approved neratinib for the extended adjuvant treatment of patients with early stage, HER2-positive breast cancer following postoperative trastuzumab on July 17, 2017.
APHINITY
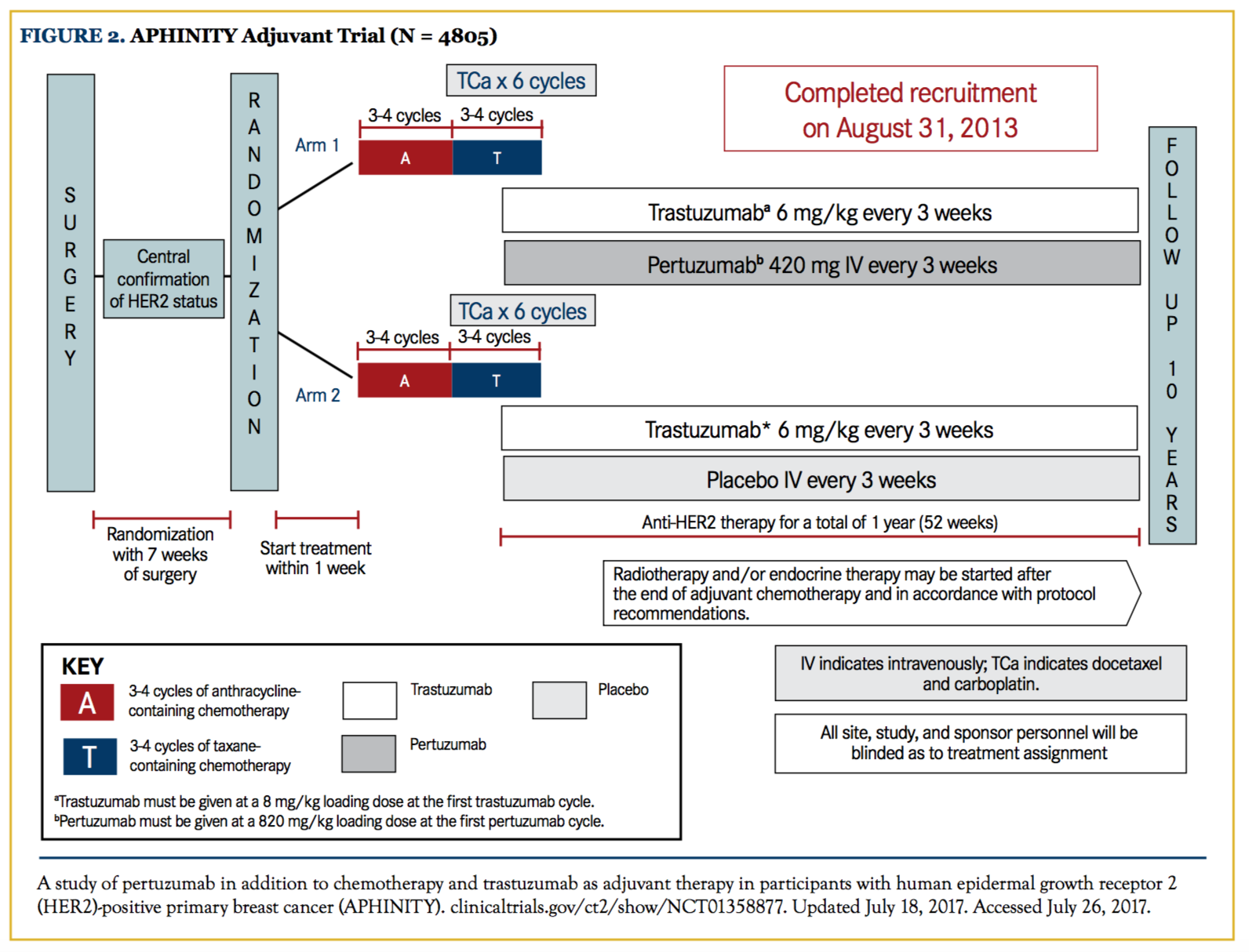
This phase III randomized clinical trial included 4805 patients with operable HER2-positive breast cancer, and evaluated the efficacy and safety of pertuzumab plus trastuzumab and chemotherapy compared with trastuzumab and chemotherapy as adjuvant therapy. Patients with tumors smaller than 1 cm were excluded. Overall, 63% of patients had node-positive disease, and 36% had HR-negative disease. At an early follow-up of 3 years, 93.2% of patients who received trastuzumab alone had not developed recurrent disease, compared with 94.1% of those who received pertuzumab and trastuzumab. However, the reported 4-year iDFS favored the pertuzumab arm, 92.3% versus 90.6%, with a difference of 1.7% (hazard ratio, 0.81) that was statistically significant. When evaluating results by nodal status, the benefit was greater in patients with node-positive disease (89.9% vs 86.7%), with the difference of 3.2% favoring the pertuzumab arm. No treatment effect was noted in the node-negative group24,25 (Figure 2).
TEACH
This placebo-controlled, randomized phase III trial enrolled 3161 patients who previously received adjuvant chemotherapy but not trastuzumab (either because they were treated before the adjuvant use became standard, or in a country where trastuzumab was not available) who were randomized to receive lapatinib or placebo for 12 months. About a quarter of patients had finished their treatment nearly 4 years ago. For patients who received adjuvant lapatinib versus placebo, there was no significant difference in the 4-year DFS (87% vs 83%, respectively) or OS (94% vs 94%) between the groups.26
Future Directions
ATEMPTTrastuzumab emtansine (T-DM1) is an approved therapy for metastatic HER2-positive breast cancer and has demonstrated a survival benefit. It is also extremely well tolerated. The ATEMPT trial is evaluating if chemotherapy can be omitted in stage I HER2-positive breast cancer. Patients are randomized to either T-DM1 or paclitaxel in combination with trastuzumab, followed by 1 year of trastuzumab.27 If positive, this study could establish T-DM1 as an effective and well tolerated (nonalopecia-causing) regimen in the adjuvant setting for selected patients.
KATHERINE
This study is evaluating the use of adjuvant T-DM1 versus trastuzumab in patients who did not achieve a pathologic complete response after neoadjuvant therapy. This study, if positive, could establish adjuvant T-DM1 for patients with residual tumor after neoadjuvant therapy.28
ATOP
Elderly patients (60 years and older) have a higher risk of toxicity from chemotherapy, necessitating development of less toxic regimens. This study is testing T-DM1 for older patients with stage I to III HER2-positive breast cancer who decline or are not candidates for standard chemotherapy.29
NSABP B-47
Central testing of patients included in the NSABP and NCCTG adjuvant trials demonstrated that HER2-negative patients derived similar benefits from trastuzumab that have been demonstrated in HER2-positive patients. Accordingly, this randomized phase III study tested the hypothesis that trastuzumab can be effective in patients with node-positive or high-risk node-negative breast cancer who are “HER2-low” (1+ or 2+ by immunohistochemistry, not amplified by fluorescence in situ hybridization). These patients were randomized to chemotherapy with or without trastuzumab for 1 year, with a primary aim to determine if the addition of trastuzumab improves DFS.30 Results are eagerly awaited.
Conclusions
There is room to further improve outcomes for patients with HER2-positive breast cancer who receive treatment in the adjuvant setting. With the results of the APHINITY and the ExteNET trials, there are now data to support the addition of concurrent pertuzumab for high-risk patients or the sequential use of neratinib in HR-positive patients after a year of trastuzumab. However, it will be interesting to see if some practitioners will choose a hybrid approach—to combine both concurrent pertuzumab and sequential neratinib for selected patients despite lack of any data supporting this strategy. The main issues of adding these therapies will be the cost and adverse effects, to get a small but statistical significant improvement in iDFS and no difference in OS. It would be important in the future to find the subgroup of patients who benefit the most from the addition of these therapies. Unfortunately, despite the demonstrated improvements in many studies, a percentage of patients continue to relapse. We also continue to remain concerned with the toxicities of therapies. There is evidence that anthracyclines may be omitted without compromising efficacy. Longer durations of treatment with trastuzumab (HERA), and the addition of bevacizumab (BETH) or lapatinib (ALLTO), have not proved to be beneficial. Neratinib, when given following completion of 1 year of adjuvant trastuzumab, demonstrated improvement in iDFS, with the benefit limited to HR-positive patients. Adding 1 year of pertuzumab in APHINITY appeared to add a modest benefit in iDFS for node-positive patients. Future efforts should remain focused on identifying biomarkers for improving patient selection to be able to successfully exclude those who are unlikely to benefit from these expensive interventions while we continue to improve our treatment regimens. Author affiliations: Reshma Mahtani, DO; Ana Sandoval, MD; and Mohammad Jahanzeb, MD, are all with the Miller School of Medicine, University of Miami, Sylvester Comprehensive Cancer Center. Address correspondence to: Reshma Mahtani, DO, University of Miami, 1192 East Newport Center Dr, Deerfield Beach, FL 33442. E-mail: [email protected]. Financial disclosures: Reshma Mahtani, DO, has received research grant support from Genentech.
References
- Moasser MM, Krop IE. The evolving landscape of HER2 targeting in breast cancer. JAMA Oncol. 2015;1(8):1154-1161. doi: 10.1001/jamaoncol.2015.2286.
- Coussens L, Yang-Feng TL, Liao YC, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985;230(4730):1132-1139.
- Schechter AL, Stern DF, Vaidyanathan L, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312(5994):513-516.
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177-182.
- Cameron D, Piccart-Gebhart MJ, Gelber RD, et al; Herceptin Adjuvant (HERA) Trial Study Team. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195-1205. doi: 10.1016/S01406736(16)32616-2.
- Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744-3752. doi: 10.1200/JCO.2014.55.5730.
- Slamon DJ, Eierman W, Robert NJ, et al. Ten-year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab in HER2-positive early breast cancer. Paper presented at: 2015 San Antonio Breast Cancer Symposium; December 11, 2015; San Antonio, TX.
- Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27(34):56855692. doi: 10.1200/JCO.2008.21.4577.
- Spielmann M, Roché H, Delozier T, et al. Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol. 2009;27(36):6129-6134. doi: 10.1200/JCO.2009.23.0946.
- O’Sullivan CC, Bradbury I, Campbell C, et al. Efficacy of adjuvant trastuzumab for patients with human epidermal growth factor receptor 2-positive early breast cancer and tumors ≤2 cm: a meta-analysis of the randomized trastuzumab trials. J Clin Oncol. 2015;33(24):2600-2608. doi: 10.1200/JCO.2015.60.8620.
- Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27(34):5700-5706. doi: 10.1200/ JCO.2009.23.2025.
- Vaz-Luis I, Ottesen RA, Hughes ME, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol. 2014;32(20):2142-2150. doi: 10.1200/JCO.2013.53.1608.
- Pivot X, Romieu G, Debled M, et al; PHARE trial investigators. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14(8):741-748. doi: 10.1016/S1470-2045(13)70225-0.
- Conte PF, Bisagni G, Frassoldati A, et al. 9 weeks vs 1 year adjuvant trastuzumab in combination with chemotherapy: results of the phase III multicentric Italian study Short-HER. J Clin Oncol. 2017;35(suppl; abstr 501).
- Jones SE, Collea R, Paul D, et al. Adjuvant docetaxel and cyclophosphamide plus trastuzumab in patients with HER2-amplified early stage breast cancer: a single-group, open-label, phase 2 study. Lancet Oncol.2013;14(11):1121-1128. doi: 10.1016/S14702045(13)70384-X.
- Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer [published correction appears in N Engl J Med. 2015;373(20):1989]. N Engl J Med. 2015;372(2):134-141. doi: 10.1056/NEJMoa1406281.
- Tolaney SM, Barry WT, Guo H, et al. Seven-year (yr) follow-up of adjuvant paclitaxel (T) and trastuzumab (H) (APT trial) for node-negative, HER2-positive breast cancer (BC). J Clin Oncol. 2017;35(suppl; abstr 511).
- Moreno-Aspitia A, Holmes EM, Jackisch C, et al. Updated results from the phase III ALTTO trial (BIG2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T ➔L) or their combination (L+T) in the adjuvant treatment of HER2-positive early breast cancer. J Clin Oncol. 2017;35(suppl; abstr 502).
- Slamon DJ, Swain SM, Buyse M, et al. Primary results from BETH, a phase 3 controlled study of adjuvant chemotherapy and trastuzumab ± bevacizumab in patients with HER2-positive, node-positive or high-risk node-negative breast cancer. Paper presented at: 2013 San Antonio Breast Cancer Symposium; December 11, 2013; San Antonio, TX.
- Chan A, Delaloge S, Holmes FA, et al. Neratinib after adjuvant chemotherapy and trastuzumab in HER2-positive early breast cancer: primary analysis at 2 years of a phase III, randomized, placebo-controlled trial (ExteNET). J Clin Oncol. 2015;33(suppl; abstr 508).
- Chan A, Delaloge S, Holmes FA, et al; ExteNET Study Group. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17(3):367-377. doi: 10.1016/S1470-2045(15)00551-3.
- Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in early-stage HER2-positive breast cancer: 3-year analysis from a phase 3 randomized, placebo-controlled, double-blind trial (ExteNET). 2015 San Antonio Breast Cancer Symposium; December 8-12, 2015; San Antonio, TX.
- FDA Briefing Document. Oncologic Drugs Advisory Committee Meeting. May 24, 2017. NDA 208051. Applicant: Puma Technology, Inc. FDA website. fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM559721.pdf. Accessed June 15, 2017.
- von Minckwitz G, Procter MJ, de Azambuja E, et al. APHINITY trial (BIG 4-11): a randomized comparison of chemotherapy (C) plus trastuzumab (T) plus placebo (Pla) versus chemotherapy plus trastuzumab (T) plus pertuzumab (P) as adjuvant therapy in patients (pts) with HER2-positive early breast cancer (EBC). J Clin Oncol. 2017;35(suppl; abstr LBA500).
- von Minckwitz G, Procter M, de Azambuja E, et al; APHINITY Steering Committee and Investigators. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017 [Epub ahead of print]. doi: 10.1056/NEJMoa1703643.
- Goss PE, Smith IE, O’Shaughnessy J, et al; TEACH investigators. Adjuvant lapatinib for women with early-stage HER2-positive breast cancer: a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(1):88-96. doi: 10.1016/S1470-2045(12)70508-9.
- T-DM1 vs paclitaxel/trastuzumab for breast (ATEMPT Trial). NCT01853748. clinicaltrials.gov/ct2/show/NCT01853748. Updated August 24, 2016. Accessed April 10, 2017.
- A study of trastuzumab emtansine versus trastuzumab as adjuvant therapy in patients with HER2-positive breast cancer who have residual tumor in the breast or axillary lymph nodes following preoperative therapy (KATHERINE). clinicaltrials. gov/ct2/show/NCT01772472. Updated June 14, 2017. Accessed July 20, 2017.
- Trastuzumab emtansine in treating older patients with human epidermal growth factor receptor 2-positive stage I-III breast cancer. NCT02414646. Updated May 5, 2017. Accessed July 20, 2017. Chemotherapy with or without trastuzumab after surgery in treating women with invasive breast cancer. NCT01275677. clinicaltrials.gov/show/NCT01275677. Updated June 28, 2017. Accessed July 20, 2017.
- Chemotherapy with or without trastuzumab after surgery in treating women with invasive breast cancer. NCT01275677. clinicaltrials.gov/show/NCT01275677. Updated June 28, 2017. Accessed July 20, 2017.