Introduction
Anti-CDK4/6 agents inhibit the phosphorylation of the retinoblastoma (Rb) tumor suppressor, which promotes Rb-E2F binding and prevents E2F-mediated oncogenic transcription. Slamon and colleagues showed compelling preclinical data indicating the efficacy of palbociclib in estrogen receptor positive (ER+) breast cancer cell lines. These experiments established that in the absence of hormonal therapy, palbociclib is cytostatic, and that when combined with estrogen blockade, there is a synergistic decrease in cell proliferation.
The phase II PALOMA-1 trial demonstrated a 10-month improved progression-free survival (PFS) in women with ER+ MBC treated with first-line letrozole plus palbociclib, a CDK4/6 inhibitor, versus letrozole alone (20.2 vs 10.2 months, hazard ratio [HR], 0.488, one-sided P = .0004). This led to the larger phase III PALOMA-2 trial, which confirmed a 10-month improved PFS in women with ER+ MBC treated with first-line letrozole plus palbociclib versus letrozole alone (24.8 vs 14.5 months, HR, 0.58; P
<.000001). Importantly, these trials enrolled women who had not received endocrine therapy for their metastatic disease.
The phase III PALOMA-3 trial demonstrated a 5-month improved PFS in women with ER+ MBC who had progressed despite endocrine therapy for their metastatic disease, and were treated with palbociclib plus fulvestrant, versus fulvestrant alone (9.5 vs 4.6 months; HR, 0.46; P <.0001). Together, these studies have elevated palbociclib plus letrozole as the preferred first-line therapy in women with ER+ MBC, and palbociclib plus fulvestrant as an effective therapy in patients with ER+ MBC not previously treated with palbociclib who have progressed on a nonsteroidal aromatase inhibitor.
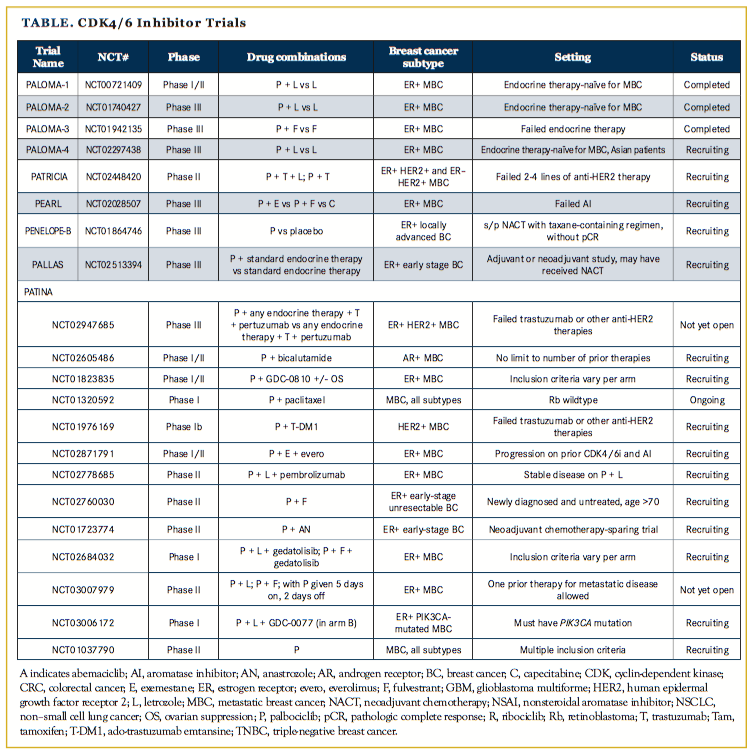
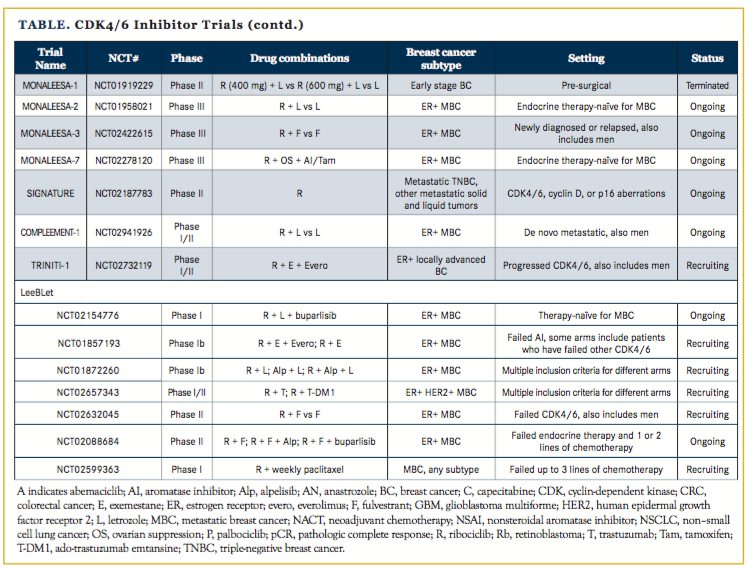
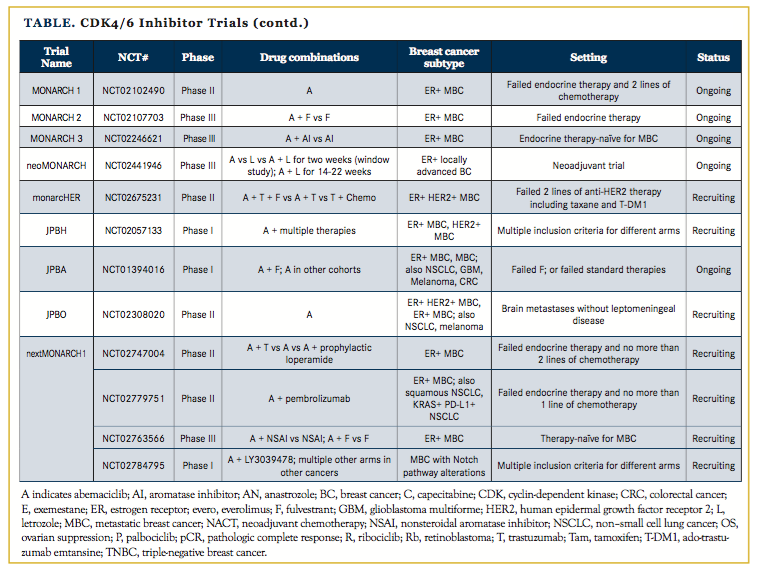
The success of palbociclib has spurred the development of other CDK4/6 inhibitors including ribociclib, which is now FDA approved in combination with fulvestrant, and abemaciclib, which has been granted an FDA breakthrough therapy designation. Numerous clinical trials are investigating these CDK4/6 inhibitors in settings beyond metastatic disease (Table), including adjuvant and neoadjuvant trials and novel combinations with other targeted therapies and immunotherapies. Therefore, it will be of great interest to see where these drugs show efficacy and if there may be differential activities among the inhibitors.
CDK4/6 Inhibitors and Clinical Profiles
Palbociclib has a half-maximal inhibitory concentration (IC50) for CDK4/6 of 9 to 15 μM.8 The most frequent adverse events (AEs)of palbociclib are neutropenia, thrombocytopenia, and fatigue. The most frequent grade 3/4 AEs are pulmonary embolism (4%) and diarrhea (2%). Palbociclib is dosed at 125 mg twice daily, 3 weeks on and 1 week off. Importantly in clinical trials, few patients had febrile neutropenia.
Ribociclib has an IC50 for CDK4/6 of 11 to 39 μM.8 The most frequent AEs with ribociclib are neutropenia, nausea, and thrombo- cytopenia. The most frequent grade 3/4 AEs are neutropenia and thrombocytopenia. Of note, aspartate aminotransferase/alanine aminotransferase increases (15%) and corrected QT interval prolongation were observed (8%); therefore, serial liver-function test monitoring and electrocardiograms are recommended when prescribing ribociclib. Ribociclib is dosed at 600 mg daily, 3 weeks on and 1 week off.
Abemaciclib has an IC50 for CDK4/6 of 2 to 5 μM,8 and it can penetrate the blood-brain barrier. The most frequent AEs of abemaciclib are neutropenia and diarrhea. Almost all patients will also have an asymptomatic creatinine increase, which is an on-target AE of abemaciclib because it inhibits renal efflux transporters in the proximal tubule of the kidney. The most frequent grade 3/4 AEs are neutropenia and diarrhea. Some clinical trials now integrate prophylactic loperamide, an antidiarrheal medication, with abemaciclib. Abemaciclib as a single agent is dosed continuously at 200 mg twice a day. In combination with endocrine therapy, abemaciclib is currently under investigation at 150 mg twice a day. Of note, abemaciclib is effective as monotherapy without the need for hormonal blockade,7 and this may be due to its increased affinity for CDK4, which is important for breast cancer oncogenesis, as compared with CDK6.
Biomarkers of Response and Resistance, Mechanisms of Sensitivity, and Mechanisms of Resistance
While palbociclib is a targeted therapy, we do not understand the characteristics of ER+ breast cancers that predict for clinical response. Palbociclib owes its development to decades of work on the cell cycle,9 which culminated in the 2001 Nobel Prize in Physiology or Medicine for Hartwell, Hunt, and Nurse.
Based on these seminal studies, one may predict that amplification of cyclin D1 (which binds to CDK4/6 and is required for its enzyme activity) or loss of p16 (which is a negative regulator of the CDK4/6-cyclin D1 complex) would enhance sensitivity to CDK4/6 inhibitors. PALOMA-1,3 which tested letrozole with and without palbociclib in patients with ER+ MBC, enrolled molecularly defined cohorts of patients with amplification of cyclin D1, loss of p16, or both. However, these tumor alterations did not predict for response to palbociclib, and ER positivity remains the only validated bio- marker of response. In addition, PALOMA-35 showed that neither PIK3CA mutational status (as detected by circulating tumor DNA) nor quantitative level of ER positivity predicted for response to palbociclib. Additional biomarker analyses from PALOMA-210 did not reveal any other cell-cycle–related genes that predicted for response to palbociclib plus letrozole.
Forty-five percent of patients on palbociclib do not derive an objective response and, among the patients who initially respond, 50% of them progress after 2 years of therapy.1 Currently, the only accepted mechanism of intrinsic resistance to CDK4/6 inhibitors in patients is Rb loss, which is rare (2.4%) in non-triple negative MBC.11 In vitro experiments have implicated cyclin E amplification,12 CDK6 amplification,13 and increased pyruvate dehydrogenase kinase 114 as mechanisms of acquired resistance to palbociclib monotherapy; how- ever, these associations with clinical resistance to CDK4/6 blockade have yet to be confirmed.
Biomarkers have also been explored in the neoadjuvant CDK4/6 inhibitor space. The NeoPalAna trial15 studied neoadjuvant anastrozole for 4 weeks, followed by the addition of palbociclib to anastrozole for four 28-day cycles, with single-agent anastrozole continuing until surgery. Biopsies were collected on starting palbociclib, after 2 weeks of palbociclib, and at surgery. There was a significantly increased rate of complete cell-cycle arrest (defined as Ki67 protein <2.7%) after 2 weeks of palbociclib plus anastrozole as compared with when starting palbociclib (87% vs 26%). How CDK4/6 inhibitors modulate Ki67 and whether or not decreased Ki67 translates into a decreased risk of recurrence and increased survival are open questions. Additional biomarker analysis showed that neither luminal breast cancer subtype nor PIK3CA mutational status predicted for response to palbociclib plus anastrozole. Palbociclib-resistant tumors had increased expression of cell-cycle genes CCND3, CCNE1, and CDKN2D on gene-expression analysis. Given that these genes are all downstream targets of the E2F1 transcription factor, it will be interesting to test to see if palbociclib resistance may be characterized by a cell-cycle gene signature.
The phase II neoMONARCH trial,16 which investigates neoadjuvant abemaciclib plus anastrozole, includes a “window study” in which patients obtain a pretreatment biopsy and are initially randomized to 2 weeks of abemaciclib monotherapy, anastrozole monotherapy, or a combination of the 2, after which they receive a posttreatment biopsy. After these 2 weeks, patients are continued on abemaciclib and anastrozole for 14 to 22 weeks. The primary endpoint is a decrease in Ki67 after 2 weeks of treatment. The study met its primary endpoint and both abemaciclib monotherapy as well as abemaciclib and anastrozole combination caused decreased Ki67 as compared with anastrozole alone.
Another research team17 has shown that decreases in tumor Ki67 parallel decreases in serum thymidine kinase in patients on neoadjuvant palbociclib plus anastrozole. This may provide preclinical data for a new serum biomarker for response to CDK4/6 inhibitors, at least in the neoadjuvant arena.
Many CDK4/6 inhibitor clinical trials are collecting pre-, on-, and posttreatment biopsies, as well as circulating tumor DNA, for targeted next-generation sequencing, and these studies may reveal biomarkers or determinants of response and resistance to CDK4/6 inhibitors. In summary, other than ER-positivity, we do not understand the mechanisms of sensitivity or resistance to palbociclib and CDK4/6 inhibitors in ER+ MBC, apart from Rb loss predicting for resistance. Elucidating these resistance mechanisms will be crucial to leveraging the efficacy of CDK4/6 inhibitors.
Sequencing of Therapies and Finding a Place for Ribociclib and Abemaciclib
Currently, palbociclib is approved as first-line therapy in patients with de novo ER+ MBC, and palbociclib and ribociclib are approved for patients with recurrent metastatic disease who have progressed on endocrine therapies. Given the emergence of other CDK4/6 inhibitors, one important question is whether patients who have progressed on or after palbociclib may derive benefit from continued CDK4/6 inhibition, including treatment with palbociclib or another agent such as ribociclib or abemaciclib.
Some preclinical data suggest non–cross-resistance among CDK4/6 inhibitors. One study18 generated palbociclib- and ribociclib-resistant cell clones and showed that some of these clones were sensitive to abemaciclib. Many ribociclib trials are exploring this question, including NCT0185719319 (ribociclib plus exemestane plus everolimus, a mechanistic target of rapamycin inhibitor; or ribociclib plus exemestane), TRINITI-120 (ribociclib plus exemestane plus everolimus), and NCT0263204521 (ribociclib plus fulvestrant vs fulvestrant) in patients who have previously received a CDK4/6 inhibitor.
Palbociclib and ribociclib are similar in that both require hormonal therapy for efficacy in ER+ MBC. However, abemaciclib also has single-agent activity, as shown in the phase II MONARCH-1 trial.7 MONARCH-1 enrolled a heavily pretreated patient population after prior progression on endocrine therapy and at least 1 prior chemotherapy agent for MBC, and showed about a 20% response rate and about a 40% clinical benefit rate, including patients with stable disease, with a median overall survival of about 22 months on abemaciclib monotherapy.22 Expanding on these data, and capitalizing on the penetration of abemaciclib into the central nervous system, the JPBO trial23 is investigating single-agent abemaciclib in patients with ER+ and ER+/human epidermal growth factor receptor–positive (HER2+) brain metastases.
Another outstanding clinical question concerns the optimal therapy after a patient has progressed on first-line palbociclib plus letrozole. Current standard options are hormonal therapy plus everolimus, hormonal therapy alone, or chemotherapy (eg, capecitabine). Hope- fully, detailed correlative molecular analyses of patients on CDK4/6 inhibitor clinical trials will be able to answer this critical question, and to determine if genomically defined patient subsets (eg, ESR1 mutations, PIK3CA mutations) may respond differently.
CDK4/6 Inhibitors in Other Breast Cancer Subtypes and Settings, and With Chemotherapy and Immunotherapy
While CDK4/6 inhibitors are effective in ER+ breast cancer, it remains to be seen if they are also effective in patients with ER+/ HER2+ breast cancer or triple-negative breast cancer (TNBC).
Some trials exploring the efficacy of CDK4/6 inhibitors in combination with trastuzumab or ado-trastuzumab emtansine (T-DM1) in ER+ HER2+ MBC are PATRICIA24 (palbociclib plus trastuzumab plus letrozole vs palbociclib plus trastuzumab, also with arms for ER– HER2+ patients), NCT0197616925(palbociclib plus T-DM1), NCT02657343 (ribociclib plus trastuzumab; ribociclib plus T-DM1), monarcHER26 (abemaciclib plus trastuzumab plus fulvestrant vs abemaciclib plus trastuzumab vs trastuzumab plus chemotherapy of physician’s choice), and JPBO20 (abemaciclib monotherapy).
While CDK4/6 inhibitors were initially thought to have improved efficacy in TNBC, preclinical work did not support this hypothesis, although there were some TNBC cell lines that did have adequate IC50s for palbociclib. CDK4/6 inhibitors are the subject of multiple trials in the metastatic TNBC space including NCT0260548627 (palbociclib plus bicalutamide) in androgen-receptor–positive patients, SIGNATURE28 (ribociclib monotherapy), NCT0259936329 (ribociclib plus weekly paclitaxel) in Rb-wildtype patients of any subtype, JPBA30 (arm with abemaciclib monotherapy), and NCT0278479531 (abemaciclib plus Notch inhibitor LY3039478) in patients with Notch pathway alterations of any subtype.
Since many chemotherapeutics (eg, taxanes) require an intact cell cycle, combining these therapies may or may not be synergistic. Pre-clinical data supporting the combination of CDK4/6 inhibitors with chemotherapy are mixed, suggesting either lack of cytotoxic synergy with CDK4/6 inhibitors32 or attenuation of CDK4/6 inhibitor-induced cytotoxicity.33 Some phase I clinical trials are exploring combining CDK4/6 inhibitors with cytotoxic chemotherapy, including NCT0259936326 (ribociclib plus weekly paclitaxel). Palbociclib plus weekly paclitaxel34 can be administered safely, and we await trials exploring efficacy of these combinations.
Checkpoint blockade immunotherapy is a clear success in melanoma, non–small cell carcinoma, and other solid tumors; however, its role in breast cancer is not clear. An arm of KEYNOTE 01235 (pembrolizumab, a monoclonal antibody against PD-1) showed that of 32 patients with heavily pretreated PD-L1+ metastatic TNBC, there was a 19% response rate and a 26% clinical benefit rate. An arm of KEYNOTE 02836 (pembrolizumab) demonstrated a 12% response rate and 20% clinical benefit rate in heavily pretreated patients with ER+ HER2– PD-L1+ MBC. As in other solid tumors, PD-L1 positivity is predictive but not prognostic of response to checkpoint blockade. Low response rates in MBC have also been observed in the JAVELIN trial37 (avelumab, a monoclonal antibody against PD-L1).
The modest response of checkpoint blockade in ER+ MBC has spurred clinical trials looking at ways to potentiate immunotherapy with CDK4/6 inhibition. NCT02779751,38 a phase II clinical trial, is evaluating the safety and preliminary efficacy of abemaciclib plus pembrolizumab. NCT02778685,39 another phase II clinical trial, is investigating the safety and preliminary efficacy of adding pembrolizumab to palbociclib plus letrozole in patients with stable disease on palbociclib plus letrozole. These studies and their correlative biomarkers may reveal ways to potentiate the modest efficacy of checkpoint blockade in ER+ MBC.
Conclusion
The addition of palbociclib to the armamentarium of therapies in ER+ MBC is of great utility for patients; however, many fundamental questions remain about biomarkers for response and resistance, the role of next-generation CDK4/6 inhibitors, efficacy in other breast cancer subtypes, and combinations with other targeted therapies, chemotherapy, and immunotherapy. Multidisciplinary work integrating basic science, translational science, and clinical trials will be required to leverage fully the potential of CDK4/6 inhibitors in patients.
Author affiliations: Neil Vasan and Maura N. Dickler are with Memorial Sloan Kettering Cancer Center, New York, NY.
Send correspondence to: Maura N. Dickler, MD, Breast Medicine Service, Memorial Sloan Kettering Cancer Center, Weill Cornell Medical Center, 300 East 66th St, New York, NY 10065, e-mail: [email protected].
Disclosures and Funding: Neil Vasan is a recipient of the NCI MSK T32 Investigational Cancer Therapeutics Training Program Grant (T32-CA009207).
Author disclosures: None.
- Lim S, Kaldis P. CDKs, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140(15):3079-3093. doi: 10.1242/ dev.091744.
- Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. doi: 10.1186/bcr2419.
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35. doi: 10.1016/S1470- 2045(14)71159-3.
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925-1936.
- Christofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425-439. doi: 10.1016/S1470-2045(15)00613-0.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first- line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738-1748.
- Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH1: Results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. J Clin Oncol. 34; 2016 (suppl; abstr 510).
- Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14(2):130-146. doi: 10.1038/nrd4504.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674. doi: 10.1016/j.cell.2011.02.013. Review.
- Finn R, Jiang Y, Rugo H, et al. Biomarker analyses from the phase 3 PALOMA-2 trial of palbociclib (P) with letrozole (L) compared with placebo (PLB) plus L in postmenopausal women with ER plus /HER2–advanced breast cancer (ABC). Ann Oncol. 2016; 27 (suppl_6): LBA15. doi: 10.1093/annonc/mdw435.05.
- Trere D, Brighenti E, Donati G, et al. High prevalence of retinoblastoma protein loss in triple-negative breast cancers and its association with a good prognosis in patients treated with adjuvant chemotherapy. Ann Oncol. 2009;20(11):1818-1823. doi: 10.1093/ annonc/mdp209.
- Herrera-Abreu MT, Palafox M, Asghar U, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76(8):2301-2313. doi: 10.1158/0008-5472.CAN-15-0728.
- Yang C, Li Z, Bhatt T, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene. 2016. doi: 10.1038/ onc.2016.379
- Jansen VM, Formisano L, Witkiewicz A, et al. PI3K/PDK1 mediates resistance to CDK4/6 inhibitors through dysregulation of S-phase cyclins/cyclin dependent kinases (CDKs). Cancer Res. 2017; (77) (suppl 4): P3-03-05. doi: 10.1158/1538-7445.
- Ma CX, Gao F, Luo J, et al. NeoPalAna: Neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor positive breast cancer. Clin Cancer Res. 2017. pii: clincanres.3206.2016. doi: 10.1158/1078-0432.CCR-16-3206.
- Hurvitz S, Martin M, Abad MF, et al. Biological effects of abemaciclib in a phase 2 neoadjuvant study in postmenopausal patients with HR+, HER2- breast cancer. Paper presented at: 40th Annual San Antonio Breast Cancer Symposium; December 8, 2016; San Antonio, TX; https://www.sabcs.org/Program/Daily-Schedule/Day-3. Accessed April 6, 2017.
- Liu N, Thomas S, Luo R, et al. Serum thymidine kinase 1 activity as a pharmacodynamics marker of cyclin-dependent kinase 4/6 inhibition in patients with early stage breast cancer receiving neo- adjuvant palbociclib. Paper presented at: 40th Annual San Antonio Breast Cancer Symposium; December 9, 2016; San Antonio, TX. doi: 10.1158/1538-7445.
- Lenihan C, Bouchekioua-Bouzaghou K, Abdulghani R, Chupin J, Shia A, Schmid P. CDK4/6 inhibitor resistant ER-positive cells remain dependent on estrogen signaling and retain sensitivity to endocrine therapy. Paper presented at: 40th Annual San Antonio Breast Cancer Symposium; December 8, 2016; San Antonio, TX. doi: 10.1158/1538-7445.
- Phase Ib trial of LEE011 with everolimus (RAD001) and exemestane in the treatment of hormone receptor positive HER2 negative advanced breast cancer. (2017). ClinicalTrials.gov. https://clinical- trials.gov/ct2/show/NCT01857193?term=NCT01857193&rank=1. (Identification No. NCT01857193). Accessed April 2017
- Study of ribociclib with everolimus plus exemestane in HR+ HER2- locally advanced/metastatic breast cancer post progression on CDK 4/6 inhibitor. (TRINITI-1). (2017). ClinicalTrials. gov. https://clinicaltrials.gov/ct2/show/NCT02732119?ter- m=NCT02732119&rank=1. (Identification No. NCT02732119). Accessed April 2017.
- Study of efficacy of ribociclib after progression on CDK4/6 inhibition in patients with HR+ H2N- advanced breast cancer. (2017). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/ NCT02632045?term=NCT02632045&rank=1. (Identification No. NCT02632045). Accessed April 2017.
- Rugo HS, Tolaney SM, Cortes J, et al. MONARCH 1: Final overall survival analysis of a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. Paper presented at: 110th American Association for Cancer Research; April 3, 2017; Washington, DC. Abstract.
- A study of abemaciclib (LY2835219) in participants with breast cancer, non¬-small cell lung cancer, or melanoma that has spread to the brain. (2017). ClinicalTrials.gov. https:// clinicaltrials.gov/ct2/show/NCT02308020?term=A+- study+of+abemaciclib+AND+LY2835219+AND+in+participants+with+breast+cancer%2C+non%E2%80%93small+cell+lung+- cancer%2C+or+melanoma+that+has+spread+to+the+brain&rank=1. (Identification No. NCT02308020). Accessed April 10, 2017.
- Study of palbociclib and trastuzumab with or without letrozole in HER2-positive metastatic breast cancer (PATRICIA). (2017). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/ NCT02448420?term=Study+of+Palbociclib+and+Trastuzum- ab+With+or+Without+Letrozole+in+HER2-positive+Metastatic+Breast+Cancer+%28PATRICIA%29&rank=1. (Identification No. NCT02448420). Accessed April 10, 2017.
- Phase 1b Study of PD-0332991 in combination with T-DM1(trastuzumab-DM1) (2017). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01976169?term=NCT01976169&rank=1. (Identification No. NCT01976169). Accessed April 10, 2017.
- A study of abemaciclib (LY2835219) in women with HR+, HER2+ locally advanced or metastatic breast cancer (monarcHER) (2017). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/ NCT02308020?term=NCT02308020&rank=1. (Identification No. NCT02675231). Accessed April 10, 2017.
- Palbociclib in combination with bicalutamide for the treatment of AR(+) metastatic breast cancer (MBC) (2017). ClinicalTrials. gov. https://clinicaltrials.gov/ct2/show/NCT02605486?ter- m=NCT02605486&rank=.1 (Identification No. NCT02605486). Accessed April 10, 2017.
- LEE011 for patients with CDK4/6 pathway activated tumors (SIGNATURE) (2017). ClinicalTrials.gov. https://clinicaltrials.gov/ ct2/show/NCT02187783?term=NCT02187783&rank=1. (Identifica- tion No. NCT02187783). Accessed April 10, 2017.
- A trial of ribocilcib (LEE011) and weekly paclitaxel in patients with Rb+ advanced breast cancer (2017). ClinicalTrials. gov. https://clinicaltrials.gov/ct2/show/NCT02599363?ter- m=NCT02599363&rank=1. (Identification No. NCT02599363). Accessed April 10, 2017.
- A phase 1 study of LY2835219 in participants with advanced can- cer (2017). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/ NCT01394016?term=NCT01394016&rank=1. (Identification No. NCT01394016). Accessed April 10, 2017.
- A study of LY3039478 in participants with advanced or metastatic solid tumors (2017). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/ show/NCT02784795?term=NCT02784795&rank=1. (Identification No. NCT02784795). Accessed April 10, 2017.
- McClendon AK, Dean JL, Rivadeneira DB, et al. CDK4/6 inhibition antagonizes the cytotoxic response to anthracycline therapy. Cell Cycle. 2012;11(14):2747-2755. doi: 10.4161/cc.21127.
- Roberts PJ, Bisi JE, Strum JC, et al. Multiple roles of cyclin-de- pendent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104(6):476-487. doi: 10.1093/jnci/djs002.
- Clark AS, O’Dwyer P, Troxel A, et al. Palbociclib and paclitaxel on an alternating schedule for advanced breast cancer: Results of a phase Ib trial. [SABCS abstract P6-13-08]. Cancer Res. 2016;76(suppl 4):Abstract P6-13-08. doi: 10.1158/1538-7445.
- Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEY- NOTE-012 study. J Clin Oncol. 2016;34(21):2460-2467. doi: 10.1200/ JCO.2015.64.8931.
- Rugo HS, Delord JP, Im SA, et al. Preliminary efficacy and safety of pembrolizumab (MK-3475) in patients with PD-L1–positive estrogen receptor–positive (ER+)/HER2–negative advanced breast cancer enrolled in KEYNOTE-028. Paper presented at: 110th American Association for Cancer Research; December 11, 2015. San Antonio, TX. Abstract S5-07. doi: 10.1158/1538-7445.
- Dirix LY, Takacs I, Nikolinakos P, et al. Avelumab (MS- B0010718C), an anti–PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase Ib JAVELIN solid tumor trial. Paper presented at: 110th American Association for Cancer Research; December 9, 2015. San Antonio, TX. Abstract S1-04. doi: 10.1158/1538-7445.
- A study of abemaciclib (LY2835219) in participants with non-small cell lung cancer or breast cancer (2017). ClinicalTrials. gov. https://clinicaltrials.gov/ct2/show/NCT02779751?ter- m=NCT02779751&rank=1. (Identification No. NCT02779751). Accessed April 10, 2017.
- Pembrolizumab, letrozole, and palbociclib in treating patients with stage IV estrogen receptor positive breast cancer with stable dis- ease that has not responded to letrozole and palbociclib (2017). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02778685?ter- m=NCT02778685&rank=1. (Identification No. NCT02778685). Accessed April 10, 2017.