Introduction
In the recurrent metastatic breast cancer (MBC) setting, a growing consensus encourages retesting the receptor status of metastatic tumors. However, there is scant evidence to suggest that changing first-line treatment plans based on the status improves clinical outcomes. In fact, evidence exists that changes to first-line treatment plans can harm patient outcomes.1-3 Decades of research surrounding this topic exists, yet the most important question has not yet been addressed:
When receptors are discordant, should first-line treatment plans rely on the receptor status of the primary tumor or on the receptor status of the metastatic tumor?
Approximately 6000 US women a year are diagnosed with discordant receptor results. These patients and their physicians must choose between disparate treatment plans indicated by the discordance.3 Currently, insufficient research-based evidence exists to inform guidelines for national and international standards when discordance occurs.
The purpose of this research was to determine the impact on patient outcomes in recurrent MBC diagnoses with discordant receptors when the first-line treatment plan was based on the receptor status of metastatic tumors instead of the receptor status of the primary tumors.
Methods
Overview
A thorough review of research published prior to November 2014 on MBC tumor retesting was performed. From that analysis, a hypothesis was formed: In discordant cases, if the first-line treatment plan is based on the receptor status of the primary tumor, the median life expectancy of patients with MBC will be longer than those whose first-line treatment plan is based on the receptor status of the metastatic tumor. We designed a retrospective observational study testing this hypothesis. This study queried the Tumor Registry at the University of Tennessee Cancer Center. All patients in the registry with recurrent MBC from January 1, 2000, to September 30, 2014, were considered. Proven endocrine and targeted therapies were available by 2000 for all patients in the study.4 Institutional Review Board permission was granted by the Graduate School of Medicine and the University of Tennessee.
Study Decision Flow
Figure 1 outlines the study protocol. This study screened patients with a recurrence of MBC and determined whether they needed receptor status retesting (cohorts A and B in Figure 1).
Retested patients were grouped by whether their primary and metastatic tumor receptor statuses were concordant or not (C and D). Discordant patients were further parsed by whether their first-line medical treatment plan was based on the primary or the metastatic tumor receptor status. These 2 groups are represented as cohorts E and F in Figure 1.
The study’s main objective was to compare the treatment plan impact of cohorts E and F. The literature review revealed multiple studies that compared cohorts C and D,1,2,5-14 and 1 study compar- ing groups A and B,6 as depicted in Figure 1. No research explicitly had a protocol to compare the survival rates of cohorts E and F.
The literature that evaluated the clinical impact of discordance did so by comparing the survival of patients with concordant versus discordant tumors (cohorts C and D). These studies revealed that cohort D patients are always, to some degree, confounded with cohort F. Our analysis is unique because this confounding effect is removed by specifically comparing the survival curves of groups E and F.
Patient Outcomes
In this study, patient outcomes were measured by: (1) 5-year postrecurrence survival (PRS) time currence to death or censoring, and (2) the first-line follow-up scan results using the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1) standard.15
Phenotypes and Discordance
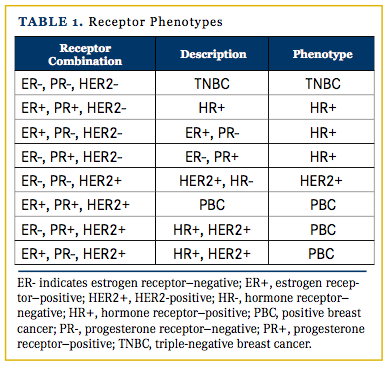
The term “discordance” was strictly defined as a difference in the primary tumor receptor status (phenotype) and the metastatic tumor phenotype that warranted a change in treatment plan based on current National Comprehensive Cancer Network standards.16 The 4 phenotypes in this study were triple-negative breast cancer (TNBC), hormone receptor positive (HR+ and HER2-negative), HER2-positive (HER2/neu+ and HR-negative), and positive breast cancer (PBC) (Table 1). Dieci et al8 also used these 4 phenotypes in their research.
Data Parameters
Based on our protocol, we identified the variables necessary to conduct the research. Variable information was extracted from The University of Tennessee Cancer Center Tumor Registry and the electronic medical records and paper charts of the study’s patients. Patients having MBC with recurrences between January 1, 2000, and November 1, 2014, were reviewed. HER2 testing was not common until 2000.
Statistical Methods
Statistical methods were determined a priori. To compare the survival data of cohorts E and F, the log-rank test for Ka- plan-Meier survival curves was used. Cox regression analysis was utilized for multivariate analyses. Chi-square analyses were completed on the RECIST v1.1 data. Univariate tests were reported on available covariates using t-tests and chi-square analysis. SAS JMP Pro version 10.0 was used for all analyses.
Results
Patient Characteristics
Of 317 patient records evaluated, 124 met the study protocols established and provided complete information. Many records included in the search were not recurrences of MBC and/or had extensive missing data.3 Out of 124 patients, 92 had tumor retesting, with 14 receptor status results discordant with their primary tumors. Eight patients had their first-line treatment plan based on their primary tumor receptor status and, 6 had the plan based on the tumor receptor status of their metastatic tumors. The sampling breakdown is shown in Figure 1.
Treatment Strategy Survival and First-Scan Results
Our study compared clinical outcomes of patients with MBC with discordant results on whether their first-line treatment plans were based on the primary or metastatic tumor receptor status. The results of this comparison showed that survival rates for patients whose first-line treatment plans were based on the receptor status of their primary tumors (n = 8) were better than patients whose plans were based on the metastatic tumor receptor status (n = 6; 48 vs 8.4 months, respectively; P = .049; Figure 2).
Our findings demonstrated that patients with recurrent MBC with treatment plans using the receptor status of their primary tumors had a median survival of 48 months versus 8 months for patients whose plans were based on the metastatic receptor status. This sample difference of a 40-month life expectancy was consistent with the results of Liedtke et al,1 who evaluated the impact of discordance only.
Chi-square analysis based on the RECIST v1.1 standard was not statistically significant (P = .164). However, the results are consistent with our hypothesis, given that: (1) of the 5 patients who had progressive disease at the first rescan, 4 were treated based on their metastatic tumor receptors; (2) 5 of the 7 patients with stable disease were treated based on their primary tumors; and (3) the only patient with a partial response was treated based on primary tumor receptor status.
Univariate Analysis
A 2-sample univariate analysis was performed on each available covariate for the 14 discordant patients. This tested whether statistically significant differences existed between patients with treatment plans based on their primary tumor receptor status and those with treatment plans based on the metastatic tumor (Table 2). Multivariate analyses of 124 patients yielded 6 statistically significant covariates.3 (see asterisks in Table 2). The fact that no univariate results were statistically significant was not unexpected, given the combined sample size of 14.
The visceral nature (location) of the metastatic disease did present some concerns in the univariate analysis. All patients with treatment plans based on their metastatic tumor receptor status had metastatic tumors rated as visceral, while only 4 of 8 patients with first-line treatment plans based on their primary tumor were rated visceral. Thus, visceral and metastatic treatment plans were statistically confounded. None of the 14 patients were deemed to be in visceral crisis at the time of metastatic diagnosis. Two evaluations were performed to assess the confounding impact: (1) Cox regression analysis on the 14 discordant patients and (2) Kaplan-Meier survival analysis on visceral patients only. Both tests compared the impact of first-line treatment plans on survival outcomes. Each analysis yielded results consistent with the overall findings of our study.3 The univariate and multivariate analyses provided evidence that the covariate confounding did not impact our study conclusions.
Discussion
Clinical practice guideline recommendations are provided by 3 standards organizations regarding treatment of metastatic patients with discordant tumor receptor status.16,18,19 Although each standard contains slight variations in verbiage and caveats, all suggest considering the metastatic tumor receptor status to assist in determining first-line treatment plans for recurrent MBC. No references to published research are provided to support the guidelines. Two standards clearly indicate that no data are available to support their recommendations. Both our analysis and literature review contradict these guidelines, instead advocating that MBC treatment decisions be based on the receptor status of the primary tumor.
Retesting Metastatic Tumors
The literature review consistently supported the importance of retesting metastatic tumors for confirmation of diagnosis of MBC20-23 and to assess discordance between the receptor status of the primary and metastatic tumors.2,8,24-26 A recent meta-analysis by Aurilio et al25 summarized 48 articles that involved 3000 to 4000 tumors. They found discordance rates of 20%, 33%, and 8% for estrogen receptor, progester-one receptor, and HER2, respectively.
Several authors, directly or indirectly, explored the impact that retesting tumor receptors had on first-line treatment plan determination, estimating the percentage of treatment plan decisions based upon the metastatic tumor status when it was discordant with the primary tumor. Five articles8,24,27-29 found that first-line treatment plan decisions were influenced 50% to 70% of the time by the existence of discordance, with the more recent studies trending to a higher percentage.
Clinical Outcomes of Discordance
Researchers investigated the clinical outcomes of patients with discordant receptors focusing on life expectancy without directly testing the effect of changing treatment plans based on discordance.7,9,10,12-14 Our careful analysis of several Discussion sections yielded evidence that changing treatment plans based on the metastatic tumor receptor status was more harmful than helpful.3 A strong illustration of this evidence is found in Liedtke et al,1 where the inappropriate use of targeted therapies due to discordance was discussed as a potential cause of poor survival. The authors implied that patients with discordant receptor status were often treated contrary to what the primary tumor status would indicate and that this change likely contributed to poor life expectancy. The authors reported that the median PRS rate for patients with TNBC with concordant receptors was 43 months. Patients with TNBC (primary tumor) whose metastatic tumor receptors were discordant experienced a 15.6-month median PRS.1 The complete evidence in the literature and a discussion of potentially contradictory data can be found in Pannell.3
The challenge of evaluating research on discordance, its impact on treatment decisions, and the subsequent effect on survival outcomes was that discordance and treatment plan determinations were confounded. This was highlighted in Turner and Di Leo’s23 literature review of the prognostic impact of discordance. Our study is the first designed to address this confounding and to estimate the impact that first-line treatment plan decisions have on the clinical outcomes.
Research Limitations
Our sample size is statistically small. Although several publications investigating discordance had small sample sizes,1,2,7,14 our study’s requirement to further divide discordant cases impacts the statistical precision of our analysis. In retrospective observational studies, evaluating the potential effect of covariates is important. While our covariate analysis found no evidence for potential bias, it remains possible that unknown confounding effects exist.
Future Research
Future research should increase the sample size and breadth of this study, engaging additional researchers and cancer centers. Additionally, the exact causes of discordance have been speculated about but not definitively determined.21 The ultimate question of interest is: Why would determining first-line treatment plans based on the primary tumor receptor status, as opposed to the metastatic tumor, result in better patient outcomes? The only answer proposed in our literature review was by Liedtke et al,1 who provided a discussion of the potential impact of measurement error in testing metastatic tumor receptor status in patients with MBC. Foukakis et al21 further discussed measurement error; Sighoko et al30 attempted to measure the impact of measurement error on discordance; and Heofnagel et al31 discussed the variation of receptor measurements between different metastases in the same patient.
Conclusions and Recommendations
The evidence in this research is consistent with our hypothesis. Ultimately, the study showed that patients with MBC who had discordant results and first-line treatment plans based on their primary tumors rather than their metastatic tumors had a longer median life expectancy of 40 months. This evidence is supported by: a thorough literature review of historical data, a log-rank test with a P value of .049, a covariate analysis, and a sensitivity analysis.3
Since 1989, the clinical impact of testing metastatic tumors for receptor status to inform first-line treatment plans has been studied.32 Prior to our research, no definitive conclusions regarding the impact of retesting receptor status on the clinical outcomes of patients with MBC had been drawn. Our research goal is to influence changes in international and national standards regarding determination of first-line treatment plans in discordant cases of MBC and to provide a higher level of evidence for those standards. Based on our research, we propose the following:
Where discordance between the primary and metastatic tumor receptor status would indicate different treatments, the status of the primary tumor should take precedence when developing the first-line treatment plan for a patient with newly diagnosed, recurrent metastatic breast cancer. Strong clinical evidence to the contrary must be present to warrant basing the treatment plan on the metastatic tumor receptor status.
Our recommendation is made with a suggested level of evidence of 2B.
Author Affiliations: Dr. Pannell is from the Department of Business Analytics, Lincoln Memorial University, Knoxville, TN; Dr. Panella is from the University of Tennessee Medical Center at Knoxville, Graduate School of Medicine, Department of Internal Medicine; and Dr. Zaretzki is from the Department of Analytics and Statistics, The University of Tennessee, Knoxville.
Corresponding Author: T. Allen Pannell, Jr, PhD, Department of Business Analytics, Lincoln Memorial University, 601 W. Summit Hill Drive, Knoxville, TN 37902;. e-mail: [email protected].
Author Disclosures: The authors report no conflicts of interest to disclose. No funding was provided for this research.


REFERENCES
- Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009;20(12):1953-1958. doi: 10.1093/annonc/mdp263.
- Niikura N, Liu J, Hayashi N, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol. 2012;30(6):593-599. doi: 10.1200/ JCO.2010.33.8889.
- Pannell TA. The metastatic receptor status impact on first-line treatment plans and outcomes for recurrent metastatic breast cancer [PhD dissertation, University of Tennessee, 2015].
- Harries M, Smith I. The development and clinical use of trastuzumab (Herceptin). Endocr Relat Cancer. 2002;9(2):75-85.
- Amir E, Miller N, Geddie W, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;30(6):587- 592. doi: 10.1200/JCO.2010.33.5232.
- Botteri E, Disalvatore D, Curigliano G, et al. Biopsy of liver metastasis for women with breast cancer: impact on survival. Breast. 2012;21(3):284-288. doi: 10.1016/j. breast.2011.12.014.
- Chang HJ, Han SW, Oh DY, et al. Discordant human epidermal growth factor receptor 2 and hormone receptor status in primary and metastatic breast cancer and response to trastuzumab. Jpn J Clin Oncol. 2011;41(5):593-599. doi: 10.1093/jjco/hyr020.
- Dieci MV, Barbieri E, Piacentini F, et al. Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: a single-institution analysis. Ann Oncol. 2013;24(1):101-108. doi: 10.1093/annonc/mds248.
- Duchnowska R, Dziadziuszko R, Trojanowski T, et al; Polish Brain Metastasis Consortium. Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res. 2012;14(4):R119. doi: 10.1186/bcr3244.
- Idirisinghe PK, Thike AA, Cheok PY, et al. Hormone receptor and c-ERBB2 status in distant metastatic and locally recurrent breast cancer. Pathologic correlations and clinical significance. Am J Clin Pathol. 2010;133(3) 416-429. doi: 10.1309/AJCPJ57FLLJRXKPV.
- Karlsson E, Appelgren J, Solterbeck A, Bergenheim M, Alvariza V, Bergh J. Breast cancer during follow-up and progression - a population based cohort on new cancers and changed biology. Eur J Cancer. 2014;50(17):2916-2924. doi: 10.1016/j.ejca.2014.08.014.
- Lower EE, Glass E, Blau R, Harman S. HER-2/neu expression in primary and metastatic breast cancer. Breast Cancer Res Treat. 2009;113(2):301-306. doi: 10.1007/s10549008-9931-6.
- Montagna E, Bagnardi V, Rotmensz N, et al. Breast cancer subtypes and outcome after local and regional relapse. Ann Oncol. 2012;23(2):324-331. doi: 10.1093/annonc/ mdr129.
- Wilking U, Karlsson E, Skoog L, et al. HER2 status in a population-derived breast cancer cohort: discordances during tumor progression. Breast Cancer Res Treat. 2011;125(2):553-561. doi: 10.1007/s10549-010-1029-2.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RE- CIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228- 247. doi: 10.1016/j.ejca.2008.10.026.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology - Breast Cancer. 2014. Version 3.2014. www.NCC.org.
- Breast cancer. American Cancer Society, 2015 (Cancer Information Database).
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd International Consensus Guidelines for Advanced Breast Cancer (ABC2). Ann Oncol. 2014:25(10):1871-1888. doi: 10.1093/annonc/mdu385.
- Van Poznak C, Somerfield MR, Bast RC, et al. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2015;33(24):2695-2704. doi: 10.1200/JCO.2015.61.1459.
- Criscitiello C, André F, Thompson AM, et al. Biopsy confirmation of metastatic sites in breast cancer patients: clinical impact and future perspectives. Breast Cancer Res. 2014;16(2):205.
- Foukakis T, Aström G, Lindström L, Hatschek T, Bergh J. When to order a biopsy to characterise a metastatic relapse in breast cancer. Ann Oncol. 2012;23(suppl 10):x349-x353.
- Penault-Llorca F, Coudry RA, Hanna WM, Osamura RY, Rüschoff J, Viale G. Experts’ opinion: recommendations for retesting breast cancer metastases for HER2 and hormone receptor status. Breast. 2013;22(2):200-202. doi: 10.1016/j.breast.2012.12.004.
- Turner NH, Di Leo A. HER2 discordance between primary and metastatic breast cancer: assessing the clinical impact. Cancer Treat Rev. 2013;39(8):947-957. doi: 10.1016/j. ctrv.2013.05.003.
- Amir E, Clemons M, Purdie CA, et al. Tissue confirmation of disease recurrence in breast cancer patients: pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat Rev. 2012;38(6):708-714. doi: 10.1016/j. ctrv.2011.11.006.
- Aurilio G, Disalvatore D, Pruneri G, et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer. 2014;50(2):277-289. doi: 10.1016/j.ejca.2013.10.004.
- Farolfi A, Ibrahim T, Scarpi E, Amadori D. Biology matters: the clinical impact of single-receptor discordance on breast cancer. Ann Oncol. 2013;24(3):851. doi: 10.1093/an-nonc/mdt005.
- Aurilio G, Monfardini L, Rizzo S, et al. Discordant hormone receptor and human epidermal growth factor receptor 2 status in bone metastases compared to primary breast cancer. Acta Oncol. 2013;52(8):1649-1656. doi: 10.3109/0284186X.2012.754990.
- Lindström LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30(21):2601-2608. doi: 10.1200/JCO.2011.37.2482.
- Thompson AM, Jordan LB, Quinlan P, et al; Breast Recurrence in Tissues Study Group Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res. 2010;12(6):R92. doi: 10.1186/ bcr2771.
- Sighoko D, Liu J, Hou N, Gustafson P, Huo D. Discordance in hormone receptor status among primary, metastatic, and second primary breast cancers: biological difference or misclassification? Oncologist. 2014;19(6):592-601. doi: 10.1634/theoncologist.2013-0427.
- Hoefnagel LD, van der Groep P, van de Vijver MJ, et al; Dutch Distant Breast Cancer Metastases Consortium. Discordance in ERα, PR and HER2 receptor status across different distant breast cancer metastases within the same patient. Ann Oncol. 2013;24(12):3017-3023. doi: 10.1093/ annonc/mdt390
- Kamby C, Rasmussen BB, Kristensen B. Oestrogen receptor status of primary breast carcinomas and their metastases. Relation to pattern of spread and survival after recurrence. Br J Cancer. 1989;60(2):252-257.