Introduction
Metaplastic carcinoma accounts for 0.25% to 1% of annual breast malignancies diagnoses. Primary metaplastic squamous cell carcinoma (SCC) of the breast is a very rare breast malignancy, accounting for less than 0.1% of all breast carcinomas.1 SCC was first reported in 1908, and diagnosis is established when more than 90% of the malignant cells are of squamous cell origin.2 Squamous cell carcinoma is considered to be more aggressive compared with other infiltrating ductal cancers, and knowledge regarding treatment patterns and outcomes has been limited.2-4 To confirm a diagnosis of primary SCC of the breast, the following conditions must be fulfilled: (1) absence of an associated primary SCC in a second site, (2) absence of skin involvement, and (3) there must be a clear predominance (>90%) of areas with SCC at histologic examination.2 Clinical and radiographic characteristics are not specific, and tumors are usually estrogen receptor (ER)–, progesterone receptor (PR)–, and human epidermal growth factor receptor 2 (HER2)-negative.2,5 As management options are still limited, the prognosis and optimal treatments remain controversial. In this report, we will present a case of this rare breast malignancy.
Case Presentation
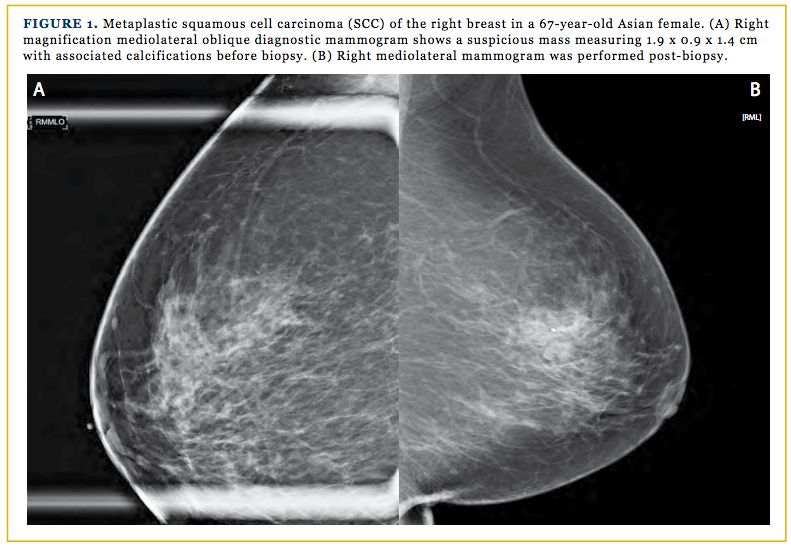
A 67-year-old previously healthy Asian woman was referred to our clinic for a newly diagnosed right breast cancer. The patient stated that prior to the screening mammogram, she had no pain, swelling, or nipple discharge. She also never had breast abnormality on mammogram; therefore, she never had a biopsy of any kind. She denied any family history of breast cancer, colon cancer, or ovarian cancer. The patient has no history of skin cancer, nor did she have any skin, oral pharynx, or anal lesions. Per the patient, her very first mammogram was done in 2011, which showed normal results. The most recent screening mammogram was done on October 2, 2015, which found asymmetrical breast parenchyma within the upper-outer anterior right breast, without prior studies for comparison. Her assessment after this screening was BI-RADS 0. A diagnostic mammogram was ordered, and the findings showed a suspicious mass measuring 1.9 x 0.9 x 1.4 cm with associated calcifications in the right breast at the 11:00 position, 3 cm from the nipple with an assessment of B-RADS 4 (Figures 1A and 1B).
The patient then underwent an ultrasound-guided vacuum-assisted core needle biopsy. Pathology results from the biopsy showed metaplastic SCC that was ER-positive, PR-negative, and HER2/neu-negative. The patient was then referred to our clinic for surgical evaluation. Physical examination revealed a benign bilateral exam with no palpable masses.
Right simple mastectomy with sentinel lymph node biopsy and 2 19F Jackson-Pratt drain placements were performed. Gross examination revealed a 2.4-x-2.0-x-1.3–cm firm, pink-tan, lobulated mass within the central, slightly lateral aspect of the right breast specimen. The specimen was confirmed to be a single-focus, invasive SCC (metaplastic carcinoma), with a total Nottingham score of 9/9. Areas of local invasion and necrosis can be observed microscopically in Figures 2A and 2B. Microscopic examination of the mass demonstrated SCC with keratinization (Figure 2). The margins and regional lymph nodes were negative. Ductal carcinoma in situ and lymph-vascular invasion were not identified. Additional findings were florid usual duct epithelial hyperplasia, biopsy site change, and skin with seborrheic keratosis and scar. There was no involvement of the skin, nipple, or skeletal muscle. Two sentinel nodes were biopsied and were negative for carcinoma. Pathology staging of the specimen was pT2pN0.
At the 1-week, postoperative follow-up visit, the patient appeared to be alert and not in distress. She and the oncologist discussed that adjuvant chemotherapy treatment would be beneficial given the nature and rarity of the diagnosis. The patient has completed 4 cycles of docetaxel plus cyclophosphamide, with a baseline computed tomography scan and bone scan at the time this study was written. At the 1-year follow-up visit, the patient reported that she was doing well and was currently taking letrozole, and reports little to no side effects. The 1-year follow-up screening mammogram shows scattered areas of fibroglandular density with no suspicious malignancy findings present.
Discussion
Breast cancer remains the most commonly diagnosed cancer among women, with approximately 182,000 diagnosed annually in the United States alone. Metaplastic breast carcinoma is an adenocarcinoma characterized by a histologic combination of mesenchymal and epithelial cell origin. It accounts for less than 1% of all breast cancers and is considered to be relatively rare compared with invasive ductal carcinoma.6.7 Many theories are involved in the histogenesis of SCC of the breast; however, it still remains unclear. The mean age at presentation of SCC of the breast is 54 years. The 5-year disease-free rate can be estimated around 40%, with an overall survival rate for metaplastic breast cancer to be in the range of 49% to 68%.7
Breast SCC tends to be a relatively large, rapidly growing mass that can become palpable within 2 to 3 weeks (range, 2-5 cm; median, 4 cm).2,8,9 However, in this case, the mass was never palpable on self-exam or upon physical examination. There also are no unique radiographic imaging features found for metaplastic SCC of the breast. A metaplastic carcinoma mass typically is either circumscribed or presents as an indistinct, very dense mass— with infrequent calcifications.3,10,11
More than 90% of metaplastic SCC of the breast is considered triple-negative (TNBC), which is generally expected as most SCC of the breast has the basal-like molecular subtype.2.9.12 The main characteristics of TNBC have shown similarities to basal-like cancers, including an increased frequency in younger patients (age <50 years) and a greater prevalence among African-American women, with the majority presenting as interval cancers and being significantly more aggressive than tumors of other molecular subtypes.13 However, basal-like and TNBC are not synonymous.
The management of metaplastic breast cancer has largely paralleled that of invasive ductal carcinoma management, despite growing research and clinical support that metaplastic breast cancers lie more along the spectrum of basal-like breast cancers. Patients with metaplastic TNBC receiving identical chemotherapy regimens as patients with invasive ductal carcinoma showed a poor 3-year disease-free survival rate.7,14,15 In this case, the patient was PR and HER2/neu-negative but ER-positive, which has been associated with improved relapse-free survival.2 This improvement in survival may derive from the increased range of treatment options, namely ER-targeted therapy.
The case patient is receiving adjuvant chemotherapy with docetaxel in combination with cyclophosphamide, based on experiences derived from other reports.16,17 She is currently being treated with an aromatase inhibitor after completion of adjuvant chemotherapy, because of her ER-positivity, which is in concordance with another case study.12 However, another report showed that 7 of 9 patients who received adjuvant chemotherapy developed recurrent disease. Therefore, a standard regimen for invasive ductal carcinoma may be not be effective for metaplastic breast carcinoma. However, those patients who have received adjuvant chemotherapy have demonstrated a better survival rate than those who did not.18,19
In conclusion, the rarity of primary metaplastic SCC of the breast and the aggressiveness of this disease have led to poor outcomes in response to chemotherapeutic regimens. Therefore, as standard chemotherapy and hormonal therapy approaches have not proven to be optimal in the treatment of metaplastic breast cancer, further laboratory research is needed. Clinical studies further comparing outcomes and treatment possibilities with invasive ductal carcinoma may not be feasible due to the rarity of the disease.
Patient Consent: Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author Contributions: VK conceived the idea of the report and co-wrote the manuscript; GR reviewed the literature and co-wrote the manuscript; PD performed the right simple mastectomy with sentinel lymph node biopsy; PD also interpreted the findings, critically revised the manuscript, and gave the final approval of the version to be published. All authors read and approved the final version of the manuscript.
Acknowledgments: The authors would like to thank Daniel M. Garland, MD, and Cynthia Sile, MD, for their assistance and expertise. The authors also thank RC, on whom the case report is based.
Corresponding Author: Philip D. Ding, MD, FACS, 2790 Godwin Blvd, Suite 305, Suffolk, VA 23434; E-mail: pdding@ sentara.com.
Affiliations: Ms. Konkankit and Mr. Reusser are from Saint James School of Medicine, Anguilla; and Philip Ding, MD, is with the Department of Surgery, Sentara Obici Hospital, Suffolk, VA.

REFERENCES
- Hoda SA, Brogi E, Koerner F, Rosen PP. Rosen’s Breast Pathology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014.
- Hennessy BT, Krishnamurthy S, Giordano S, et al. Squamous cell carcinoma of the breast. J Clin Oncol. 2005;23(31):7827-7835.
- Yerushalmi R, Hayes MM, Gelmon KA. Breast carcinoma—rare types: review of the literature. Ann Oncol. 2009;20(11):1763-1770. doi: 10.1093/annonc/mdp245.
- Behranwala KA, Nasiri N, Abdullah N, Trott PA, Gui G. Squa- mous cell carcinoma of the breast: clinico-pathologic implications and outcome. Eur J Surg Oncol. 2003;29(4):386-389.
- Tayeb K, Saâdi I, Kharmash M, et al. Primary squamous cell carcinoma of the breast. Report of three cases. Cancer Radiother. 2002;6(6):366-368.
- Schwartz TL, Mogal H, Papageorgiou C, Veerapong J, Hsueh E. Metaplastic breast cancer: histologic characteristics, prognos- tic factors and systemic treatment strategies. Exp Hematol Oncol. 2013;2(1):31. doi: 10.1186/2162-3619-2-31.
- Shah DR, Tseng WH, Martinez SR. Treatment options for metaplastic breast cancer. ISRN Oncol. 2012;2012:706162. doi: 10.5402/2012/706162.
- Siegelmann-Danieli N, Murphy TJ, Meschter SC, Stein ME, Prichard J. Primary pure squamous cell carcinoma of the breast. Clin Breast Cancer. 2005;6(3):270-272.
- Mitra B, Pal M, Debnath S, Saha TN, Maiti A. Primary squa- mous cell carcinoma of breast with ipsilateral axillary lymph node metastasis: an unusual case. Int J Surg Case Rep. 2011;2(7):194-197. doi: 10.1016/j.ijscr.2011.06.006.
- Günhan-Bilgen I, Memiş A, Ustün EE, Zekioglu O, Ozdemir N. Metaplastic carcinoma of the breast: clinical, mammographic, and sonographic findings with histopathologic correlation. AJR Am J Roentgenol. 2002;178(6)6:1421-1425.
- Ryckman EM, Murphy TJ, Meschter SC, Yin H. AIRP best cas- es in radiologic-pathologic correlation: metaplastic squamous cell carcinoma of the breast. RadioGraphics. 2013;33(7):2019-2024. doi: 10.1148/rg.337125189.
- Shui R, Li A, Yang F, et al. Primary squamous cell carcinoma of the breast with unusual basal-HER2 phenotype. Int J Clin Exp Pathol. 2014;7(8):5203-5209.
- Badve S, Dabbs DJ, Schnitt SJ, et al. Basal-like and triple-nega- tive breast cancers: a critical review with an emphasis on the implica- tions for pathologists and oncologists. Mod Pathol. 2011;24(2):157- 167. doi: 10.1038/modpathol.2010.200.
- Bae SY, Lee SK, Koo MY, et al. The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Breast Cancer Res Treat. 2011;126(2):471-478. doi: 10.1007/s10549-011-1359-8.
- Yanqi Z, Lina Z, Lin G. Clinicopathological features and prog- nostic factors of 22 cases of primary squamous cell carcinoma of the breast. Zhonghua Zhong Liu Za Zhi [Chinese Journal of Oncology]. 2015;37(4):293-296.
- Jones S, Holmes FA, O’Shaughnessy J, et al. Docetaxel with cy- clophosphamide is associated with an overall survival benefit com- pared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27(8):1177- 1183. doi: 10.1200/JCO.2008.18.4028.
- Jones SE, Savin MA, Holmes FA, et al. Phase III trial compar- ing doxorubicin plus cyclophosphamide with docetaxel plus cyclo- phosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24(34):5381-5387.
- Chuthapisith S, Warnnissorn M, Amornpinyokiat N, Pradni- wat K, Angsusinha T. Metaplastic carcinoma of the breast with transformation from adenosquamous carcinoma to osteosarcoma- toid and spindle cell morphology. Oncol Lett. 2013;6(3)728-732.
- Schwartz TL, Mogal H, Papageorgiou C, et al. Metaplastic breast cancer: histologic characteristics, prognostic factors and sys- temic treatment strategies. Exp Hematol Oncol. 2003;2(1):31. doi: 10.1186/2162-3619-2-31.