Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States, with an estimated 136,000 new cases and over 50,000 deaths in 2014 alone.1 As with most cancers, CRC is predominately a disease of older adults. The median age of a CRC diagnosis is 68 years, and 59% of all cases of CRC are diagnosed in persons over the age of 65 years, with 35% older than 75 years.2 As the US population continues to age, the absolute number of cancer cases and the proportion of cancers occurring in the elderly (≥65 years) will both increase.3 Thus, providing optimal care for older adults with CRC is a pressing issue.
A hallmark of aging is the gradual loss of physiologic reserve, with a resultant reduced ability to compensate when exposed to stressors such as infection, cancer, and chemotherapy.4,5 This loss of physiologic reserve is accompanied by a gradual declinein normal organ function, such as reduced cardiac motility, glomerular filtration rate, and hepatic volume.6-8 At the same time, older adults also have a shift in priorities and social support networks. Older adults are often less willing to initiate or continue treatments with severe adverse effects (AEs), and value their current time feeling well more than they value the potential for increased longevity.9,10 Thus, even when the likelihood of benefit may be the same, the AEs of adjuvant chemotherapy may be less appealing to older patients. Adjuvant treatment decisions require a careful balance of changes in physiology and priorities, with decisions being thoughtfully individualized.
Since a 1990 National Institutes of Health consensus conference, adjuvant 5-fluoropyrimidine-based chemotherapy for node-positive CRC has become the standard of care in the United States.11 However, despite these recommendations, increasing age is significantly associated with lower likelihood of receiving any adjuvant chemotherapy.12 The use of any chemotherapy drops off quickly with advancing age, with only 63% of those aged 75 to 79 years, 43% of those aged 80 to 84 years, and 14% of patients 85 years and older receiving adjuvant therapy.13 Whether this trend represents thoughtful and appropriate treatment on the part of patients and physicians based on comorbid disease and limited life expectancy or an inappropriate reflection of ageism is unclear.
Evidence for Adjuvant Therapy in Older Adults
In light of the aforementioned age-related changes, it is very reasonable for patients and physicians to question whether adjuvant therapy is equally safe and effective in the elderly. However, because only a small minority of clinical trial participants are over age 65 years, and even fewer over age 70, subset analyses by age within individual trials are underpowered.14 To overcome this challenge, multiple pooled analyses and meta-analyses have been performed. In 2001, a pooled analysis of 7 trials that randomly assigned participants to fluorouracil (5FU) with leucovorin (LV) versus observation in the adjuvant setting showed a similar beneficial treatment effect in older and younger patients.15 5FU/LV appears to be well tolerated in both the adjuvant and metastatic settings with similar grade 3 and 4 toxicity between younger and older patients with the exception of leukopenia without excess infection.15,16 These data have been confirmed in independent studies for both 5FU/LV and capecitabine.17-19
Based on the results of 3 adjuvant trials (MOSAIC,20 NSABP-C-07,21 and XELOXA22), oxaliplatin-based combinational chemotherapy is considered the standard of care for patients with stage III colon cancer, offering a 4% overall survival (OS) benefit over 5FU/LV at 6 years. However, the additional benefit of oxaliplatin in older patients appears to be attenuated. Subset analyses of MOSAIC and NSABP C-07 trials showed no significant benefit in OS with the addition of oxaliplatin in patients age 70 or older (MOSAIC mortality hazard ratio [HR] = 1.10; 95% confidence interval [CI], 0.73-1.65; for NSABP-C-07, HR = 1.32; 95% CI, 1.03-1.70).20,21 In contrast, a subgroup analysis of XELOXA, a study that evaluated the use of oral capecitabine in combination with oxaliplatin versus bolus 5FU/LV, the benefits of disease-free survival (DFS) were maintained regardless of age, but no significant OS benefit was shown.22 In an analysis using the ACCENT23 database of 2575 patients age ≥70 years using oxaliplatin-based regimens versus 5FU/LV, although there was a trend toward improved time to recurrence in oxaliplatintreated patients over age 70, there was no DFS or OS significant efficacy benefit. However, there was only a borderline significant interaction by age for OS (P = .05) and no significant interaction for DFS (P = .09), suggesting a lack of significant effect modification by age.23
Further evaluation of this in the MOSAIC subgroup saw the same trend toward prolongation of time to relapse; however, much shorter postrecurrence survival in oxaliplatintreated patients may have mitigated the benefit of any delay in recurrence.20 The benefit of oxaliplatin seems to be reduced compared with younger patients in terms of DFS and OS, while maintaining time to recurrence. A recent large population-based analysis of patients treated outside the context of a clinical trial, presumably representing a broader range of patient population than clinical trial enrollees, showed a marginal improvement in survival with the addition of adjuvant oxaliplatin in patients age 75 and older.13 Overall, these findings suggest there is minimal or no survival benefit of additional oxaliplatin to adjuvant 5FU/LV in adults age 70 or older with stage III CRC.24 In the management of patients with stage II CRC, the benefit of adjuvant chemotherapy is small regardless of age, and oxaliplatin does not improve outcomes over 5FU/LV.20
Evaluating Older Adults With Cancer
Given the potential for increased serious AEs and the long-term impacts associated with chemotherapy, the choice of whether to recommend adjuvant therapy to older adults should depend on an individual’s risk of recurrence, estimated life expectancy (without recurrence), and estimated risk of toxicity with treatment (see Table 1 for a list of useful online resources). These factors must all be considered in order to adequately weigh the risk/benefit of adjuvant treatment. Regardless of the objective measures of treatment benefit and toxicity, older patients may arrive at different personal trade-offs in terms of the benefits and risks of treatment, whether fit or frail.9 When discussing potential treatment options, it is critical to consider a patient’s values and preferences to inform the decision-making process.
Due to the heterogeneous aging process, age alone is not an adequate measure of physiologic or functional age and is a poor determinant of cancer outcomes. Traditionally, tools such as the Karnofsky Performance Status (KPS) and Eastern Cooperative Oncology Group (ECOG) Performance Status have been used by oncologists to assess functional status, but these tools are limited to simple numeric scales that although helpful, do not accurately assess the function of older patients with cancer. Many tools have been developed to better evaluate a person’s functional age. The Comprehensive Geriatric Assessment (CGA) involves a careful review of a patient’s health status, including functional status, cognition, psychological state, nutritional status, social support, polypharmacy, and comorbid illnesses (see Table 2 for more details and suggested measures).25
The feasibility of an abbreviated, primarily self-administered CGA has been shown in the oncology setting, in both community and academic outpatient clinics.26,27 CGA is able to detect impairments not typically identified in routine history or physical examination, and can be predictive of survival, chemotherapy toxicity, postoperative morbidity, and mortality in older patients with cancer 28 The CGA is a useful tool in the assessment of a patient’s overall health status and potential frailty, a state of decreased physiologic reserve.4,29 Although there is no clear consensus or definition of frailty, a number of criteria can be used to identify vulnerable older adults, such as the presence ofgeriatric syndromes (such as falls or dementia) or dependence in activities of daily living.5 Given the high risk of adverse clinical outcomes for frail older adults,30 further research is under way to better identify this population. Current evidence supports evaluating elements of the CGA as a part of a routine evaluation of older adults with cancer.28
Estimating life expectancy (without recurrence) is a critical step in assessing the potential benefit of adjuvant treatment. Several tools are available to assist in life expectancy estimation for use within a variety of different populations.31 The vast majority of CRC recurrences occur within the first 3 years, and nearly all patients with recurrences will die within 5 years. However, in patients with significant competing comorbidities that limit overall survival, 6 months of adjuvant therapy for even a 15% improvement in survival may not be warranted.32 Also, the use of Web-based tools for assessing the benefit from adjuvant therapy can help quantify outcomes with and without adjuvant therapy. Tools such as Adjuvant! Online and the calculator derived from the ACCENT database can be useful aids in the discussion of the risk of tumor recurrence and potential benefits from adjuvant chemotherapy, but they need to be interpreted in the context of physiologic age.33,34
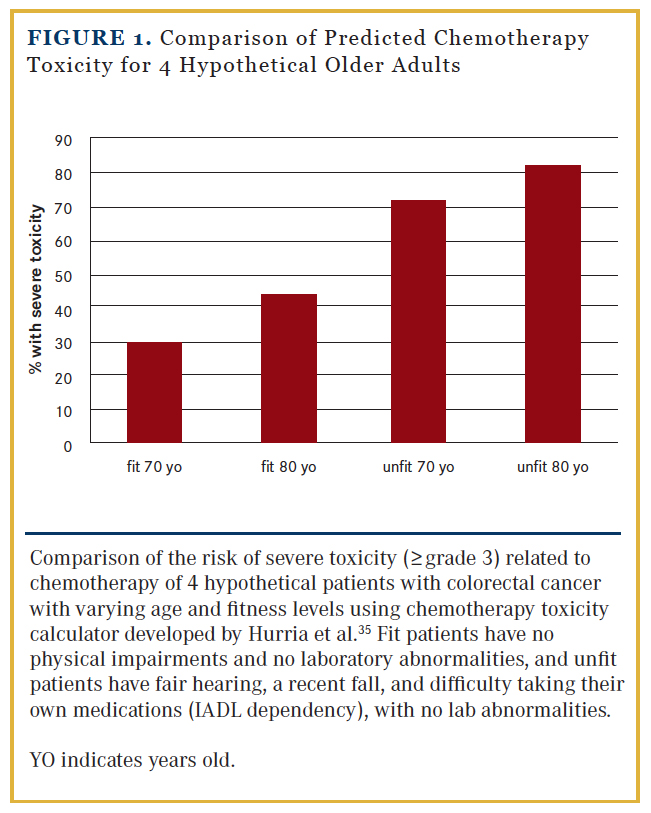
Although adjuvant chemotherapy is generally well tolerated in older adults, due to diversity in overall health and physical reserve inherent in older populations, the range of potential toxicities can vary greatly. In a seminal publication by Hurria et al,35 a chemotherapy toxicity prediction model was developed for use in older adults that incorporates features commonly known to increase risk of toxicity (age, creatinine clearance, baseline hemoglobin), as well as CGA measures such as hearing status, falls, and dependence on assistance with instrumental activities of daily living (IADL). For example, using this predictive tool, a fit 70-year-old male with no physical impairments would have an estimated 30% risk of grade 3-4 toxicity undergoing singleagent chemotherapy for CRC, whereas a similar 70-year-old male with fair hearing, a recent fall, and difficulty taking his own medications (IADL dependency) would be estimated to have a 72% risk of toxicity (Figure 1).
Although the mortality risk from adjuvant chemotherapy itself is quite low, chemotherapy is associated with AEs that may potentiate or be potentiated by other comorbidities, thus resulting in morbidity or worsened quality of life. In particular, oxaliplatin is associated with peripheral neuropathy that can be made potentially worse by preexisting diabetes or lumbar stenosis, resulting in increased risk of falls and/or disability.36
Adjuvant Treatment Approach in Older Adults With CRC
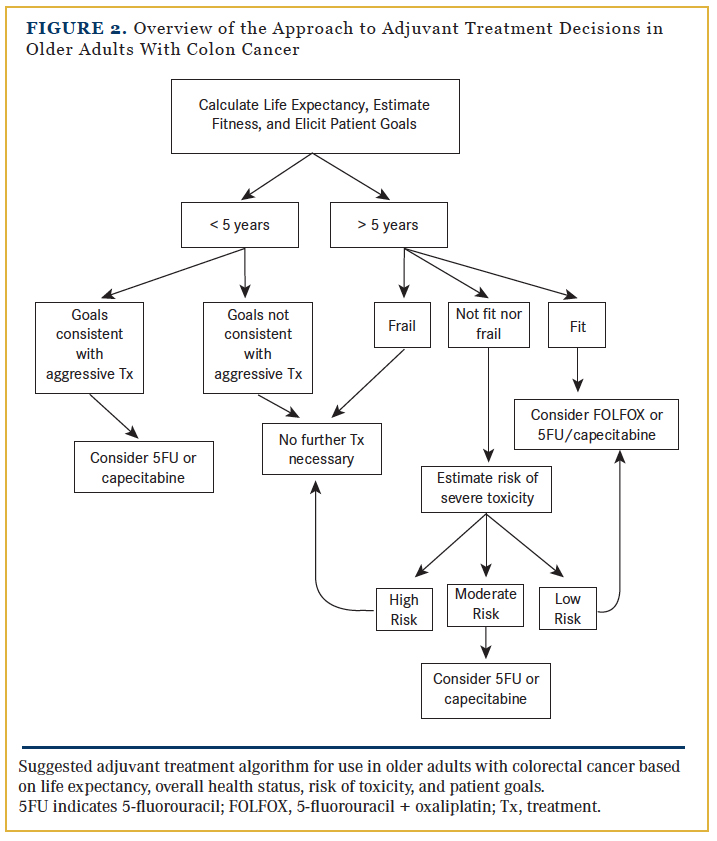
There is general agreement that fit, older adults who are active and without comorbidity and who have a life expectancy of at least 5 years should be offered adjuvant chemotherapy. Frail older adults with significant functional impairments and limited life expectancies are not suitable candidates for chemotherapy. In older adults who are neither fit nor frail, the decision-making process is the most complex and requires a delicate balance of the risks and benefits informed by patient preferences. (Figure 2 provides an overview of the approach to adjuvant treatment decisions in older patients with CRC.)
In general, we recommend adjuvant chemotherapy in all fit older patients with stage III and many with stage II high-risk CRC. For fit patients with stage III disease with life expectancies substantially greater than 5 years typically patients in their late 60s and early 70s—we suggest considering an oxaliplatin-based chemotherapy regimen, with the caveat that the absolute survival benefit is quite small. Given concern for relative 5FU/ LV resistance in tumors deficient in mismatch repair enzymes, we use FOLFOX or omit chemotherapy altogether given good prognosis. Oxaliplatin should be avoided in patients with pre-existing neuropathy, and it should be immediately stopped if significant toxicity emerges, given the uncertainty of the OS benefit of oxaliplatin-based regimens in older adults. Due to the lack of compelling benefit of oxaliplatin, we generally recommend using 5FU/LV or single-agent capecitabine. For 5FU, we recommend using the infusional modified de Gramont regimen. If an oxaliplatin-based regimen is chosen, we prefer the modified FOLFOX6 regimen, and frequently begin at 20% dose reduction and escalate subsequently if there are any concerns about tolerance. For patients with issues with count recovery, which is more frequent with older patients, we omit 5FU bolus.37,38 In patients who are unable to tolerate an ambulatory infusion pump, capecitabine plus oxaliplatin (used in the XELOX trial) is also reasonable, but we typically use 825 mg/m2 to 850 mg/m2 twice daily rather than 1000 mg/m2.22
For patients who are deemed less fit or who have comorbid conditions that are likely to limit 5-year survival, as well as those with high-risk stage II disease that are mismatch repair enzyme proficient, we recommend single-agent fluoropyrimidine. Our preference in elderly patients is for infusional 5FU rather than capecitabine, both as a single agent and in combination, in light of capecitabine-based regimens being associated with greater toxicity in older adults than infusional 5FU, at least in the metastatic setting where this has been tested.39 Eliminating the bolus of 5FU from the infusion regimen may significantly mitigate hematologic toxicity.40 Although 6 months of therapy is the optimal duration of adjuvant chemotherapy, there is evidence that 3 months of adjuvant chemotherapy may be adequate,41 so we feel comfortable discontinuing adjuvant therapy between 4 to 6 months if toxicity impairs quality of life.
Conclusions
In general, we recommend adjuvant chemotherapy in all fit older patients with stage III and stage II high-risk CRC, and recommend carefully considering the risks and benefits of adjuvant therapy in nonfit and nonfrail older adults. We also believe that it is critical to elicit the values of our older patients to better understand their preferences with regard to how they balance the potential for life prolongation and risk of AEs. Older patients receive similar benefits from 5FU-based chemotherapy as younger patients, but the incremental benefit from oxaliplatin is reduced. Treating older adults with CRC requires a carefully crafted individualized plan that balances an individual’s risk of recurrence, estimated life expectancy and risk of toxicity, and personal preferences.
Affiliations: Grant R. Williams, MD, and Hanna K. Sanoff, MD, MPH, are from the UNC Lineberger ComprehensiveCancer Center and the Department of Medicine, Division of Hematology-Oncology, University of North Carolina at Chapel Hill.
Disclosures: Dr Williams has no conflicts of interest to disclose. Dr Sanoff has received grants from Bayer and Novartis and consulting fees from Amgen.
Address correspondence to: Grant R. Williams, MD, University of North Carolina at Chapel Hill, Physician’s Office Building, 170 Manning Dr, 3rd Floor, Campus Box 7305, Chapel Hill, NC 27599-7305. Phone: 919-627-3221; fax: 919-966-6735; email: [email protected].
References
- 1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
- Surveillance Epidemiology and End Results (SEER) program. http://seer.cancer.gov/faststats/index.php. Accessed February 23, 2015.
- Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758-2765.
- Ferrucci L, Guralnik JM, Cavazzini C, et al. The frailty syndrome: a critical issue in geriatric oncology. Crit Rev Oncol Hematol. 2003;46(2):127-137.
- Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991-1001.
- Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. Western J Med. 1981;135(6):434-440.
- Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449-460.
- Sehl M, Sawhney R, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part II. Cancer J. 2005;11(6):461-473.
- Yellen SB, Cella DF, Leslie WT. Age and clinical decision making in oncology patients. J Natl Cancer Inst. 1994;86(23):1766- 1770.
- Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061-1066.
- NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264(11):1444-1450.
- Cronin DP, Harlan LC, Potosky AL, et al. Patterns of care for adjuvant therapy in a random population-based sample of patients diagnosed with colorectal cancer. Am J Gastroenterol. 2006;101(10):2308-2318.
- Sanoff HK, Carpenter WR, Sturmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol. 2012;30(21):2624-2634.
- Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383-1389.
- Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091-1097.
- D’Andre S, Sargent DJ, Cha SS, et al. 5-fluorouracil-based chemotherapy for advanced colorectal cancer in elderly patients: a North Central Cancer Treatment Group study. Clin Colorectal Canc. 2005;4(5):325-331.
- Fata F, Mirza A, Craig G, et al. Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colon carcinoma: a 10-year experience of the Geisinger Medical Center. Cancer. 2002;94(7):1931-1938.
- Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22(10):1797-1806.
- Twelves C, Scheithauer W, McKendrick J, et al. Capecitabineversus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol. 2012;23(5):1190-1197.
- Tournigand C, Andre T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol. 2012;30(27):3353-3360.
- Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29(28):3768-3774.
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29(11):1465-1471.
- McCleary NJ, Meyerhardt JA, Green E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol. 2013;31(20):2600-2606.
- Papamichael D, Audisio RA, Glimelius B, et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013 [published online July 11, 2014]. Ann Oncol. pii:mdu253.
- Hurria A. Geriatric assessment in oncology practice. J Am Geriatr Soc. 2009(57, suppl 2):S246-249.
- Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29(10):1290-1296.
- Williams GR, Deal AM, Jolly TA, et al. Feasibility of geriatric assessment in community oncology clinics. J Geriatr Oncol. 2014;5(3):245-251.
- Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol.2014. doi:10.1200/JCO.2013.54.8347.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-156.
- Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262-266.
- Yourman LC, Lee SJ, Schonberg MA, et al. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182-192.
- Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23(34):8664-8670.
- Gill S, Loprinzi C, Kennecke H, et al. Prognostic Webbased models for stage II and III colon cancer: a populationand clinical trials-based validation of numeracy and Adjuvant! Online. Cancer. 2011;117(18):4155-4165.
- Renfro LA, Grothey A, Xue Y, et al. ACCENT-based Web calculators to predict recurrence and overall survival in stage III colon cancer. J Natl Cancer Inst. 2014;106(12).
- Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465.
- Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. New Engl J Med. 2004;350(23):2343-2351.
- Hochster HS, Grothey A, Hart L, et al. Improved time to treatment failure with an intermittent oxaliplatin strategy: results of CONcePT. Ann Oncol. 2014;25(6):1172-1178.
- Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE study. J Clin Oncol. 2008;26(21):3523-3529.
- Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377(9779):1749-1759.
- Sanoff HK, Bleiberg H, Goldberg RM. Managing older patients with colorectal cancer. J Clin Oncol. 2007;25(14):1891-1897.
- Chau I, Norman AR, Cunningham D, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol. 2005;16(4):549-557.